Peeing after eating asparagus can be daunting: The liquid takes on a strange, pungent, rotten odour which can be off-putting, to say the least. But why is that?
Asparagus contains asparagusic acid which is broken down into volatile (smelly) chemicals released in the urine as soon as half an hour after consuming the vegetable. These chemical components responsible for this effect are: methanthiol, dimethyl sulfide, dimethyl disulfide, bis(methylthio)methane, dimethyl sulfoxide and dimethyl sulfone.
The really fascinating fact is that some people do not produce smelly pee after consuming asparagus (though they're rare among the population), and some people still might produce it yet not be able to smell it as offensive! Scientists have not gone into the screws & bolts of how and why this is, but the most widespread explanation is genetic variations: Some people have genes that dictate their system to process asparagusic acid somewhat differently, while variation in the smelling perception spectrum is hypothesized to be a combination of genetic and societal factors coming into play.
pic of asparagus via pellanews.gr
Showing posts with label fragrance science. Show all posts
Showing posts with label fragrance science. Show all posts
Friday, June 17, 2011
Wednesday, April 6, 2011
Perfumery Materials: Pyrazines, Burnt/Caramelised/Maple Notes
One of the new trends that's gaining momentum as we speak is the one focusing on slightly "burnt", caramelised, overcooked notes that are remininscent of toast, sticky brunt toffee or maple-laced warm milk; a darker shade of gourmand if you will! Suffice to take a look at Jeux de Peau by Serge Lutens (shades of fresh toast) or Sensuous Noir by Estée Lauder (a crème brulée almost taste under the patchouli), not forgetting Minuit Noir by Lolita Lempicka which follows the path where L de Lempicka left off. Gourmand fragrances (a subset of orientals focusing on foodie notes) aren't going anywhere; even genuine gourmet food companies are issuing their own fragrances, if Payard is anything to go by. But a more nuanced, more sophisticated approach is ushering in, hooking up even die-hard purists.
 But which materials are responsible for these flavours, these seemingly off-notes that nevertheless entice our taste buds as much as our intelligence?
But which materials are responsible for these flavours, these seemingly off-notes that nevertheless entice our taste buds as much as our intelligence?
One category is pyrazines, organic compounds with a ring structure of at least two elements. Naturally occuring in a variety of foodstuff (such as green peppers but also peas; plus they're used to enhance the "roast" factor of coffee and cured meats and to enhance the flavour of potato salad). In fragrance terms Lutens and Chris Sheldrake manipulated the roasted aroma of pyrazines into a composition that enhances the comfort factor with creamy sandalwood: eh voilà, Jeux de Peau was born!
Other molecules that render indispensable gourmand notes are:
Maltol or 3-hydroxy-2-methyl-4-pyrone...C7H8O3
.............................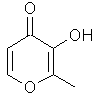
Although we have come to consider Ethylmaltol (see below) the standard "cotton candy" (candy floss) note of reference in perfumery, maltol is a naturally occuring chemical that can been found in chicory, cocoa, coffee, roasted malt, bread or even strawberry and which gives this spun caramelized effect we have come to associate with fair grounds.
Ethyl Maltol or 3-hydroxy-2-ethyl-4-pyrone...C8H10O3 ..............................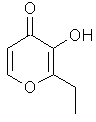
Ethyl Maltol is the ethyl analog of Maltol, of course, but this time the molecule is synthesized in the lab and is not to be found in nature: hence the boosted effect; almost 500% more than simple maltol!
Smell Thierry Mugler's Angel, the trendsetter of ethylmaltol and patchouli orientals ~with a nod to childhood~ from 1992 and be prepared to be blown away by its potent spun sugar, cotton candy note!
Furaneol(R) or 2,5-Dimethyl-4-hydroxy-3(2H)furanone ..C6H8O3
..........................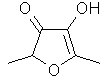
This is a molecule which was taught to samba from the craddle: it naturally contributes largely into the chemical make-up of several tropical fruit (guava, lychee, pineapple) as well as other less exotic ones (strawberry, raspberry, tomato). The fact that it is used in roasted products as well (such as corn tacos, roasted almonds, popped pop-corn or roasted coffee) contributes to its perception as a "roasting" note. I hypothesize that it's at the heart of Dior's Miss Dior Chérie, a composition based on the tension between strawberry and freshly cooked pop-corn.
Cyclotene or 3-Methyl-2-cyclopenten-2-ol-l-one ..C6H8O2
..........................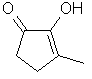
 With Cyclotene we enter the maple section of notes: Although fenugreek solid extract is used to render a maple-suryp note (indeed it was the only extract source of caramel-maple notes till the discovery of these other ingredients), actual maple suryps are further aromatized with Cyclotene; thus creating the vivid association of the molecule's odour with our perception of how maple suryp smells like! Is it maple that smells of Cyclotene or Cyclotene that smells of maple? Naturally occuring in fenugreek seeds, it's also very common today in roasted sugary products such as coffee desserts, licorice sweets, desserts with roasted almonds and, apart from fenugreek seeds, it also occurs in cocoa and coffee.
With Cyclotene we enter the maple section of notes: Although fenugreek solid extract is used to render a maple-suryp note (indeed it was the only extract source of caramel-maple notes till the discovery of these other ingredients), actual maple suryps are further aromatized with Cyclotene; thus creating the vivid association of the molecule's odour with our perception of how maple suryp smells like! Is it maple that smells of Cyclotene or Cyclotene that smells of maple? Naturally occuring in fenugreek seeds, it's also very common today in roasted sugary products such as coffee desserts, licorice sweets, desserts with roasted almonds and, apart from fenugreek seeds, it also occurs in cocoa and coffee.
Sotolon or 4,5-Dimethyl-3-hydroxy-2(5H)-furanone..C6H8O3
..........................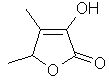
Sotolon is the key ingredient in roasted fenugreek seed and brown sugar, which is as yummy a combination as any, hence its reference as "caramel furanone" or "sugar lactone" as well as "fenugreek lactone". When it's really concentrated, it takes on curry-like tonalities while on lower concentrations it can stay within the "caramelised sugar on the pan" range of odour.
But Sotolon also possesses notes that match boozy tonalities, as it's occuring in sake, rice wine, and botrytized wine. Remember the niche fragrance Botrytis by Ginestet, meant to reproduce the "noble rot" of a fungus on the Sauternes grapes? It's got Sotolon in it, blending the pain d' épices, candied fruits and honey notes into one seamless blend.
Although Sotolon is thousands of times more powerful than Cyclotene, the modern flavours industry is using the even more powerful maple furanone (one of the most potent flavor chemicals known to man) , this time for the enhancement of the flavour of soy sauce. Thankfully, this ingredient hasn't bombasted commercial fragrances yet, but who knows what the future holds.
Related reading on Perfume Shrine: Immortelle/Helichrysum: golden sunshine of the Med , Perfumery's Raw Materials
Ref: Leffingwell Photo of maple syrup by Martin Eager
 But which materials are responsible for these flavours, these seemingly off-notes that nevertheless entice our taste buds as much as our intelligence?
But which materials are responsible for these flavours, these seemingly off-notes that nevertheless entice our taste buds as much as our intelligence?One category is pyrazines, organic compounds with a ring structure of at least two elements. Naturally occuring in a variety of foodstuff (such as green peppers but also peas; plus they're used to enhance the "roast" factor of coffee and cured meats and to enhance the flavour of potato salad). In fragrance terms Lutens and Chris Sheldrake manipulated the roasted aroma of pyrazines into a composition that enhances the comfort factor with creamy sandalwood: eh voilà, Jeux de Peau was born!
Other molecules that render indispensable gourmand notes are:
Maltol or 3-hydroxy-2-methyl-4-pyrone...C7H8O3
.............................

Although we have come to consider Ethylmaltol (see below) the standard "cotton candy" (candy floss) note of reference in perfumery, maltol is a naturally occuring chemical that can been found in chicory, cocoa, coffee, roasted malt, bread or even strawberry and which gives this spun caramelized effect we have come to associate with fair grounds.
Ethyl Maltol or 3-hydroxy-2-ethyl-4-pyrone...C8H10O3 ..............................

Ethyl Maltol is the ethyl analog of Maltol, of course, but this time the molecule is synthesized in the lab and is not to be found in nature: hence the boosted effect; almost 500% more than simple maltol!
Smell Thierry Mugler's Angel, the trendsetter of ethylmaltol and patchouli orientals ~with a nod to childhood~ from 1992 and be prepared to be blown away by its potent spun sugar, cotton candy note!
Furaneol(R) or 2,5-Dimethyl-4-hydroxy-3(2H)furanone ..C6H8O3
..........................

This is a molecule which was taught to samba from the craddle: it naturally contributes largely into the chemical make-up of several tropical fruit (guava, lychee, pineapple) as well as other less exotic ones (strawberry, raspberry, tomato). The fact that it is used in roasted products as well (such as corn tacos, roasted almonds, popped pop-corn or roasted coffee) contributes to its perception as a "roasting" note. I hypothesize that it's at the heart of Dior's Miss Dior Chérie, a composition based on the tension between strawberry and freshly cooked pop-corn.
Cyclotene or 3-Methyl-2-cyclopenten-2-ol-l-one ..C6H8O2
..........................

 With Cyclotene we enter the maple section of notes: Although fenugreek solid extract is used to render a maple-suryp note (indeed it was the only extract source of caramel-maple notes till the discovery of these other ingredients), actual maple suryps are further aromatized with Cyclotene; thus creating the vivid association of the molecule's odour with our perception of how maple suryp smells like! Is it maple that smells of Cyclotene or Cyclotene that smells of maple? Naturally occuring in fenugreek seeds, it's also very common today in roasted sugary products such as coffee desserts, licorice sweets, desserts with roasted almonds and, apart from fenugreek seeds, it also occurs in cocoa and coffee.
With Cyclotene we enter the maple section of notes: Although fenugreek solid extract is used to render a maple-suryp note (indeed it was the only extract source of caramel-maple notes till the discovery of these other ingredients), actual maple suryps are further aromatized with Cyclotene; thus creating the vivid association of the molecule's odour with our perception of how maple suryp smells like! Is it maple that smells of Cyclotene or Cyclotene that smells of maple? Naturally occuring in fenugreek seeds, it's also very common today in roasted sugary products such as coffee desserts, licorice sweets, desserts with roasted almonds and, apart from fenugreek seeds, it also occurs in cocoa and coffee.Sotolon or 4,5-Dimethyl-3-hydroxy-2(5H)-furanone..C6H8O3
..........................

Sotolon is the key ingredient in roasted fenugreek seed and brown sugar, which is as yummy a combination as any, hence its reference as "caramel furanone" or "sugar lactone" as well as "fenugreek lactone". When it's really concentrated, it takes on curry-like tonalities while on lower concentrations it can stay within the "caramelised sugar on the pan" range of odour.
But Sotolon also possesses notes that match boozy tonalities, as it's occuring in sake, rice wine, and botrytized wine. Remember the niche fragrance Botrytis by Ginestet, meant to reproduce the "noble rot" of a fungus on the Sauternes grapes? It's got Sotolon in it, blending the pain d' épices, candied fruits and honey notes into one seamless blend.
Although Sotolon is thousands of times more powerful than Cyclotene, the modern flavours industry is using the even more powerful maple furanone (one of the most potent flavor chemicals known to man) , this time for the enhancement of the flavour of soy sauce. Thankfully, this ingredient hasn't bombasted commercial fragrances yet, but who knows what the future holds.
Related reading on Perfume Shrine: Immortelle/Helichrysum: golden sunshine of the Med , Perfumery's Raw Materials
Ref: Leffingwell Photo of maple syrup by Martin Eager
Tuesday, March 22, 2011
Sense of Smell: Not as Hard-Wired as we Thought, New Study Shows
"To be a 'nose' you have to practise, just as a pianist plays his scales," said Jean-Pierre Royet, a neuroscientist at the Universite Claude Bernard in Lyon, France, and the main architect of a study published this week in the journal Human Brain Mapping. [...]Previous research had shown that constant training changes the brain activity of musicians and athletes, but no one had investigated whether the same would hold for olfaction, the ability to detect odours. To find out, Royet and colleagues enlisted 28 volunteers, half of them student perfumers, and the other half scent makers with five to 35 years in the business. [...]
"Our findings demonstrate the extraordinary ability of the brain to adapt to environmental demands and reorganise with experience," Royet said by phone.
They also show that "mental imaging of odours develops from daily practice and is not an innate skill," he added."
The very interesting article Super Sense of Smell Not Innate appears on Yahoo News page courtesy of AFP. Sandrine Videault, a New Caledonian perfumer which we have interviewed on these pages and creator of the wondrous Manoumalia is featured in the picture; a studen of E.Roudnitska, who pioneered what scienstists prove today.
Sandrine is pretty as a picture with the frangipani at arm's length, isn't she?
Thursday, February 24, 2011
Perfumery Material: Hedione (Luminous/Transparent Jasmine)
Smelling hedione, I'm struck by its beautiful, limpid and luminous character which resembles the beautiful citrusy floral note of a magnolia blossom blossoming under sunny skies.
Hedione or methyl dihydrojasmonate is an aromachemical (patented as Hedione by aroma-producing company Firmenich) that is often used in composition in substitution for jasmine absolute, but also for the sake of its own fresh-citrusy and green tonality.
 Hedione lacks the clotted cream density of natural jasmine, recalling much more the living vine in the warmth of summer mornings and for that reason it is considered a beautiful material that offers quite a bit in the production of fine perfumes.
Hedione lacks the clotted cream density of natural jasmine, recalling much more the living vine in the warmth of summer mornings and for that reason it is considered a beautiful material that offers quite a bit in the production of fine perfumes.
Perfumer Lyn Harris, nose of the brand Miller Harris and also the independent nose behind many well-known creations not credited to her name, calls it “transparent jasmine” and attributes to it the capacity to give fizz to citrus notes much “like champagne”. (See? it’s not only aldehydes which do that!)
 According to Christian Chapuis of Firmenich SA, Edouard Demole discovered methyl jasmonate in 1957, accomplished a synthesis of Hedione (from the Greek word ηδονή/hedone, meaning agreeable and pleasant) in 1958, synthesized methyl jasmonate in 1959, placed both materials under intellectual protection in 1960, and published these discoveries in 1962. "This simple timeline belies a more complex history of chemistry and creation".
According to Christian Chapuis of Firmenich SA, Edouard Demole discovered methyl jasmonate in 1957, accomplished a synthesis of Hedione (from the Greek word ηδονή/hedone, meaning agreeable and pleasant) in 1958, synthesized methyl jasmonate in 1959, placed both materials under intellectual protection in 1960, and published these discoveries in 1962. "This simple timeline belies a more complex history of chemistry and creation".
First used in the classic men’s cologne Eau Sauvage, composed by Edmond Roudnitska in 1966, hedione had been isolated from jasmine absolute and went on to revolutionize men’s scents with the inclusion of a green floral note. Eau Sauvage was so successful that many women went on to adopt it as their own personal fragrance leading the house of Dior to the subsequent introduction of Diorella in 1972, composed by the same legendary nose, blending the green floral with hints of peach, honeysuckle, rose and cyclamen in addition to the herbal citrusy notes of the masculine counterpart, all anchored by a base of cool vetiver, patchouli and oakmoss, lending a mysterious, aloof and twilit air to women who went for it.
Ten years after its introduction to perfumery, in 1976, it was the turn of Jean Claude Ellena to coax hedione in a composition that exploited its fresh and lively character to great aplomb in the production of First by jewelry house Van Cleef & Arpels (the name derived from the fact that it was their first fragrant offering, but also the first scent to come out of a jeweler too ~subsequently many followed in its tracks with notable success). In it, Ellena used 10 times the concentration of hedione used in Eau Sauvage, married to natural jasmine as well as rose de mai (rosa centifollia, which is also a "crystalline" variety), narcissus, orris, ylang ylang and a hint of carnation with the flying trapeze of aldehydes on top and the plush of vetiver, amber and vanilla at the bottom which accounted for a luminous and luxurious floral.
In it, Ellena used 10 times the concentration of hedione used in Eau Sauvage, married to natural jasmine as well as rose de mai (rosa centifollia, which is also a "crystalline" variety), narcissus, orris, ylang ylang and a hint of carnation with the flying trapeze of aldehydes on top and the plush of vetiver, amber and vanilla at the bottom which accounted for a luminous and luxurious floral.
Hedione also makes a memorable appearance in many other perfumes, such as the classic Chamade by Guerlain (introduced in 1969), Chanel no.19 (1970) and Must by Cartier (1981) and in many of the modern airy fragrances such as CKone, Blush by Marc Jacobs, the shared scent Paco by Paco Rabanne or ~surprisingly~ in the bombastic Angel by Thierry Mugler, in which it is used as a fresh top note along with helional! Perhaps if you want to feel it used in spades smell L'Eau d'Issey by Issey Miyake: the aquatic/ozonic notes cannot hide its radiance.
Its uses are legion, especially since it acts as a supreme smoothener of the rest of the ingredients. In Terre d'Hermes, perfumer Jean Claude Ellena uses lots of it to bring out the softer side of hesperidic bergamot and to fan out the woodier aspects.
High-cis Hedione is an isomer which gives a jasmine tea profile (not surprisingly, as the component naturally occurs in tea), more diffusive and less floral and thus useful in masculine blends, but it costs more than regular hedione and poses problems of stability in acidic environments. Some of the Bulgari "tea" scents, such as Bulgari Eau au Thé Rouge and Bulgari Eau au Thé Blanc are good reference points if you want to smell this in action.
Despite hedione's unlikeness to natural jasmine absolute and essential oil (which are much lusher, narcotic and indolic), perfumers have used it to supreme results in the history of fine fragrance of the last 40 years, occassionaly using it up to 35% concentrations, although it's more usual to be featured in ratios of 2-15%.
Related reading on Perfume Shrine: Perfumery Materials, The Jasmine Series
pic of jasmine via Gracemagazine. Bottle of First via zensoaps.com
Hedione or methyl dihydrojasmonate is an aromachemical (patented as Hedione by aroma-producing company Firmenich) that is often used in composition in substitution for jasmine absolute, but also for the sake of its own fresh-citrusy and green tonality.
 Hedione lacks the clotted cream density of natural jasmine, recalling much more the living vine in the warmth of summer mornings and for that reason it is considered a beautiful material that offers quite a bit in the production of fine perfumes.
Hedione lacks the clotted cream density of natural jasmine, recalling much more the living vine in the warmth of summer mornings and for that reason it is considered a beautiful material that offers quite a bit in the production of fine perfumes.Perfumer Lyn Harris, nose of the brand Miller Harris and also the independent nose behind many well-known creations not credited to her name, calls it “transparent jasmine” and attributes to it the capacity to give fizz to citrus notes much “like champagne”. (See? it’s not only aldehydes which do that!)

First used in the classic men’s cologne Eau Sauvage, composed by Edmond Roudnitska in 1966, hedione had been isolated from jasmine absolute and went on to revolutionize men’s scents with the inclusion of a green floral note. Eau Sauvage was so successful that many women went on to adopt it as their own personal fragrance leading the house of Dior to the subsequent introduction of Diorella in 1972, composed by the same legendary nose, blending the green floral with hints of peach, honeysuckle, rose and cyclamen in addition to the herbal citrusy notes of the masculine counterpart, all anchored by a base of cool vetiver, patchouli and oakmoss, lending a mysterious, aloof and twilit air to women who went for it.
Ten years after its introduction to perfumery, in 1976, it was the turn of Jean Claude Ellena to coax hedione in a composition that exploited its fresh and lively character to great aplomb in the production of First by jewelry house Van Cleef & Arpels (the name derived from the fact that it was their first fragrant offering, but also the first scent to come out of a jeweler too ~subsequently many followed in its tracks with notable success).
 In it, Ellena used 10 times the concentration of hedione used in Eau Sauvage, married to natural jasmine as well as rose de mai (rosa centifollia, which is also a "crystalline" variety), narcissus, orris, ylang ylang and a hint of carnation with the flying trapeze of aldehydes on top and the plush of vetiver, amber and vanilla at the bottom which accounted for a luminous and luxurious floral.
In it, Ellena used 10 times the concentration of hedione used in Eau Sauvage, married to natural jasmine as well as rose de mai (rosa centifollia, which is also a "crystalline" variety), narcissus, orris, ylang ylang and a hint of carnation with the flying trapeze of aldehydes on top and the plush of vetiver, amber and vanilla at the bottom which accounted for a luminous and luxurious floral.Hedione also makes a memorable appearance in many other perfumes, such as the classic Chamade by Guerlain (introduced in 1969), Chanel no.19 (1970) and Must by Cartier (1981) and in many of the modern airy fragrances such as CKone, Blush by Marc Jacobs, the shared scent Paco by Paco Rabanne or ~surprisingly~ in the bombastic Angel by Thierry Mugler, in which it is used as a fresh top note along with helional! Perhaps if you want to feel it used in spades smell L'Eau d'Issey by Issey Miyake: the aquatic/ozonic notes cannot hide its radiance.
Its uses are legion, especially since it acts as a supreme smoothener of the rest of the ingredients. In Terre d'Hermes, perfumer Jean Claude Ellena uses lots of it to bring out the softer side of hesperidic bergamot and to fan out the woodier aspects.
High-cis Hedione is an isomer which gives a jasmine tea profile (not surprisingly, as the component naturally occurs in tea), more diffusive and less floral and thus useful in masculine blends, but it costs more than regular hedione and poses problems of stability in acidic environments. Some of the Bulgari "tea" scents, such as Bulgari Eau au Thé Rouge and Bulgari Eau au Thé Blanc are good reference points if you want to smell this in action.
Despite hedione's unlikeness to natural jasmine absolute and essential oil (which are much lusher, narcotic and indolic), perfumers have used it to supreme results in the history of fine fragrance of the last 40 years, occassionaly using it up to 35% concentrations, although it's more usual to be featured in ratios of 2-15%.
Related reading on Perfume Shrine: Perfumery Materials, The Jasmine Series
pic of jasmine via Gracemagazine. Bottle of First via zensoaps.com
Wednesday, February 23, 2011
Perfumery Material: Coumarin, Tonka Bean & the Fougere accord
Open any perfume guide with fragrance "notes" or any online discussion or blog post on perfume description and you're bound to stumble on coumarin; one of the most common materials in many fine fragrances but also several body products, cosmetics and functional products. Its rich history goes back to the beginnings of modern perfumery in the late 19th century, bringing us right at the moment of the nascent concept of perfumery as a mix of organic chemistry and nature's exploitation. Coumarin as such is a synthesized material in most perfumes, but it's also found in abundance in natural products, such as tonka beans (Dipteryx odorata) where it is the principle aromatic constituent (1-3%). In fact the name derives from "cumaru", an Amazonian dialect name for the Tonka bean tree.

 The component was isolated by A.Vogel in 1820, but the laboratory synthesis of coumarin first happened in 1868 from coal tar by W.H.Perkin (who gave his name to "the Perkin reaction" used to produce it). It took another year to produce it in an industrial scale at Haarmann & Reimer. The consequent memorable inclusion of synthesized coumarin in Jicky (Guerlain 1889) and earlier in Fougère Royale (Houbigant 1882) was the kickstart of a whole new fragrance family: the fougère, thanks to Paul Parquet's composition for Houbigant. Fougère Royale contained a staggering 10% coumarin of the finished formula! How one can dream a bit while reading Guy de Maupassant describing this fragrance as "prodigious evocation of forests, of lands, not via their flora but via their greenery"...
The component was isolated by A.Vogel in 1820, but the laboratory synthesis of coumarin first happened in 1868 from coal tar by W.H.Perkin (who gave his name to "the Perkin reaction" used to produce it). It took another year to produce it in an industrial scale at Haarmann & Reimer. The consequent memorable inclusion of synthesized coumarin in Jicky (Guerlain 1889) and earlier in Fougère Royale (Houbigant 1882) was the kickstart of a whole new fragrance family: the fougère, thanks to Paul Parquet's composition for Houbigant. Fougère Royale contained a staggering 10% coumarin of the finished formula! How one can dream a bit while reading Guy de Maupassant describing this fragrance as "prodigious evocation of forests, of lands, not via their flora but via their greenery"...
The other principle constituents in the accord are lavender and oakmoss: It was only natural; lavender by itself contains coumarin in its aromatic makeup. Thus the triad comprising the main accord of the rising fougère (i.e.lavender-oakmoss-coumarin, played together like a musical chord) made coumarin itself quite popular: many classic or influential masculine colognes owe their character to it, starting of course with Jicky and continuing with Azzaro pour Homme (1978), Fahrenheit by Dior (1988), Dolce & Gabanna pour homme (1994), and Gucci pour Homme (2003).
Thus the triad comprising the main accord of the rising fougère (i.e.lavender-oakmoss-coumarin, played together like a musical chord) made coumarin itself quite popular: many classic or influential masculine colognes owe their character to it, starting of course with Jicky and continuing with Azzaro pour Homme (1978), Fahrenheit by Dior (1988), Dolce & Gabanna pour homme (1994), and Gucci pour Homme (2003).
From there coumarin infiltrated its way into many modern fragrances belonging in other families. But it was its pliability and usefulness, like a trusty Swiss knife, which made it the perfumers' darling: Are there more contrasting fragrances than the icy aldehydic Rive Gauche (YSL 1970) and the intense floral Amarige (Givenchy 1991)? Perfumers tell me that coumarin ends up in some degree in 90% of all fragrances; and in concentrations exceeding 1% it accounts for over half of the fragrances in the market!!
Coumarin conjures warm notes of tobacco (useful in masculine formulae) and because it also has caramel overtones, alternatively it can be married to vanillic components (such as vanilla, benzoin or some of the other oriental balsams, such as Tolu balsam or Peru balsam, as well as ethylvanillin) in order to play down and sophisticate their foody aspects: see it in action in orientals such as the discontinued Venezia by Laura Biagotti, Lolita au Masculin(Lempicka) or Casmir by Chopard.
In dilution coumarin projects with soft hazelnut or almond facets underneath the hay, even licorice; smell Lolita Lempicka (1997). But in higher concentration it also has spicy fresh and herbaceous facets, no doubt reminiscent of its primary role in different grasses. In combination with vanillin and bergamot, we're veering into chypre territory: Elixir des Merveilles is a no man's land with its chypre tonalities and gourmand facets.
Its versatility and its ability to "fix" smell and make it last longer allows coumarin to enter amber or woody blends (witness Samsara or Vetiver by Guerlain) as well and even heighten the appeal of spicy materials: in fact it marries very well with cinnamon or clove. Pi by Givenchy is a sweet spicy woody with lots of tonka bean, or smell L de Lolita Lempicka by Maurice Roucel. Usually, indeed coumarin is mentioned in the form of tonka beans in the traditional lists of "notes"/pyramids for fragrances (see this Index for more ingredients contributing to which "note") but it can also hide underneath grassy notes, clover, lavender, or tobacco. Modern perfumers pair it with synthetic woody-amber notes such as Kephalis and Iso-E Super to surprising results. A wonderful material indeed!
A*men (Thierry Mugler)
Amarige (Gievnchy)
Angel ~all concentrations, esp. extrait de parfum(Thierry Mugler)
Angel Sunessence (T.Mugler)
Angel La Rose (T.Mugler)
Antidote (Victor & Rolf)
Azzaro pour Homme (Loris Azzaro)
Azzaro Elixir Bois Precieux (L.Azzaro)
Blue Jeans (Versace)
Bois des Iles (Chanel)
Brit (Burberry)
Chic for Men (Carolina Herrera)
Coco (Chanel)
Coco Mademoiselle (Chanel)
Contradiction (Calvin Klein)
Etoile de Rem (Reminiscence)
Fahrenheit (Dior)
Fieno (Santa Maria Novela)
Fougere Royal (Houbigant)
Florissa (Floris)
Gloria (Cacharel)
Jasmin Noir (Bulgari)
Jicky (Guerlain)
Joop! Homme (Joop)
Kouros (Yves Saint Laurent)
Lavande (Molinard)
L de Lolita Lempicka
Lolita Lempicka (L.Lempicka)
Le Male (Jean Paul Gaultier)
Musc (Molinard)
Navy (Lily Bermuda)
Pi (Givenchy)
Rive Gauche (YSL)
Samsara (Guerlain)
Tonka Imperiale (Guerlain)
Venezia (Laura Biagotti)
Versace pour Homme (Versace)
Related reading on Perfume Shrine: Perfumery Materials one by one
source of coumarin pic via The Health Nut Corner, ad for Houbigant via Punmiris and Jicky collage via Perfumesbighouse

- Origin & function of coumarin
- History of coumarin discovery & synthesis
 The component was isolated by A.Vogel in 1820, but the laboratory synthesis of coumarin first happened in 1868 from coal tar by W.H.Perkin (who gave his name to "the Perkin reaction" used to produce it). It took another year to produce it in an industrial scale at Haarmann & Reimer. The consequent memorable inclusion of synthesized coumarin in Jicky (Guerlain 1889) and earlier in Fougère Royale (Houbigant 1882) was the kickstart of a whole new fragrance family: the fougère, thanks to Paul Parquet's composition for Houbigant. Fougère Royale contained a staggering 10% coumarin of the finished formula! How one can dream a bit while reading Guy de Maupassant describing this fragrance as "prodigious evocation of forests, of lands, not via their flora but via their greenery"...
The component was isolated by A.Vogel in 1820, but the laboratory synthesis of coumarin first happened in 1868 from coal tar by W.H.Perkin (who gave his name to "the Perkin reaction" used to produce it). It took another year to produce it in an industrial scale at Haarmann & Reimer. The consequent memorable inclusion of synthesized coumarin in Jicky (Guerlain 1889) and earlier in Fougère Royale (Houbigant 1882) was the kickstart of a whole new fragrance family: the fougère, thanks to Paul Parquet's composition for Houbigant. Fougère Royale contained a staggering 10% coumarin of the finished formula! How one can dream a bit while reading Guy de Maupassant describing this fragrance as "prodigious evocation of forests, of lands, not via their flora but via their greenery"...- The Fougere fragrance family
The other principle constituents in the accord are lavender and oakmoss: It was only natural; lavender by itself contains coumarin in its aromatic makeup.
 Thus the triad comprising the main accord of the rising fougère (i.e.lavender-oakmoss-coumarin, played together like a musical chord) made coumarin itself quite popular: many classic or influential masculine colognes owe their character to it, starting of course with Jicky and continuing with Azzaro pour Homme (1978), Fahrenheit by Dior (1988), Dolce & Gabanna pour homme (1994), and Gucci pour Homme (2003).
Thus the triad comprising the main accord of the rising fougère (i.e.lavender-oakmoss-coumarin, played together like a musical chord) made coumarin itself quite popular: many classic or influential masculine colognes owe their character to it, starting of course with Jicky and continuing with Azzaro pour Homme (1978), Fahrenheit by Dior (1988), Dolce & Gabanna pour homme (1994), and Gucci pour Homme (2003).From there coumarin infiltrated its way into many modern fragrances belonging in other families. But it was its pliability and usefulness, like a trusty Swiss knife, which made it the perfumers' darling: Are there more contrasting fragrances than the icy aldehydic Rive Gauche (YSL 1970) and the intense floral Amarige (Givenchy 1991)? Perfumers tell me that coumarin ends up in some degree in 90% of all fragrances; and in concentrations exceeding 1% it accounts for over half of the fragrances in the market!!
- The odour profile of coumarin
Coumarin conjures warm notes of tobacco (useful in masculine formulae) and because it also has caramel overtones, alternatively it can be married to vanillic components (such as vanilla, benzoin or some of the other oriental balsams, such as Tolu balsam or Peru balsam, as well as ethylvanillin) in order to play down and sophisticate their foody aspects: see it in action in orientals such as the discontinued Venezia by Laura Biagotti, Lolita au Masculin(Lempicka) or Casmir by Chopard.
In dilution coumarin projects with soft hazelnut or almond facets underneath the hay, even licorice; smell Lolita Lempicka (1997). But in higher concentration it also has spicy fresh and herbaceous facets, no doubt reminiscent of its primary role in different grasses. In combination with vanillin and bergamot, we're veering into chypre territory: Elixir des Merveilles is a no man's land with its chypre tonalities and gourmand facets.
Its versatility and its ability to "fix" smell and make it last longer allows coumarin to enter amber or woody blends (witness Samsara or Vetiver by Guerlain) as well and even heighten the appeal of spicy materials: in fact it marries very well with cinnamon or clove. Pi by Givenchy is a sweet spicy woody with lots of tonka bean, or smell L de Lolita Lempicka by Maurice Roucel. Usually, indeed coumarin is mentioned in the form of tonka beans in the traditional lists of "notes"/pyramids for fragrances (see this Index for more ingredients contributing to which "note") but it can also hide underneath grassy notes, clover, lavender, or tobacco. Modern perfumers pair it with synthetic woody-amber notes such as Kephalis and Iso-E Super to surprising results. A wonderful material indeed!
- Fragrances featuring discernible amounts of coumarin
A*men (Thierry Mugler)
Amarige (Gievnchy)
Angel ~all concentrations, esp. extrait de parfum(Thierry Mugler)
Angel Sunessence (T.Mugler)
Angel La Rose (T.Mugler)
Antidote (Victor & Rolf)
Azzaro pour Homme (Loris Azzaro)
Azzaro Elixir Bois Precieux (L.Azzaro)
Blue Jeans (Versace)
Bois des Iles (Chanel)
Brit (Burberry)
Chic for Men (Carolina Herrera)
Coco (Chanel)
Coco Mademoiselle (Chanel)
Contradiction (Calvin Klein)
Etoile de Rem (Reminiscence)
Fahrenheit (Dior)
Fieno (Santa Maria Novela)
Fougere Royal (Houbigant)
Florissa (Floris)
Gloria (Cacharel)
Jasmin Noir (Bulgari)
Jicky (Guerlain)
Joop! Homme (Joop)
Kouros (Yves Saint Laurent)
Lavande (Molinard)
L de Lolita Lempicka
Lolita Lempicka (L.Lempicka)
Le Male (Jean Paul Gaultier)
Musc (Molinard)
Navy (Lily Bermuda)
Pi (Givenchy)
Rive Gauche (YSL)
Samsara (Guerlain)
Tonka Imperiale (Guerlain)
Venezia (Laura Biagotti)
Versace pour Homme (Versace)
Related reading on Perfume Shrine: Perfumery Materials one by one
source of coumarin pic via The Health Nut Corner, ad for Houbigant via Punmiris and Jicky collage via Perfumesbighouse
Friday, November 5, 2010
Ambrox/Ambroxan: a Modern Fascination on an Elegant Material
When a new raw material enters the perfumery scene only the involved few are cognisant of the fact. When this raw material however takes the role of manna from heaven in times of crisis, however (see how synthetically-derived irones for substituting orris butter produced "the year of the iris" and how synth aoud made 2009 the year of "oud") companies invest it with panegyrics extoling its qualities. The latest material to do that is Ambrox and if you thought you haven't smelled it before think again: Almost everyone has a rather good scent memory of it through the ubiquitousness of Light Blue by Dolce & Gabbana, composed by Olivier Cresp in 2001, to name but one of the scents which use this raw material in ample amounts. In synergy with other two synthetics, Z11 and Norlimbanol, Ambrox gives Light Blue that non-perfume smooth base which made it so very popular and instantly recognisable on commuters and elevator partners across the globe.
.jpg) As is usual on Perfume Shrine when dissecting perfumery materials (this is the list with the posts on them) we revert to a little Chemistry 101 to explain Ambrox and its smell in detail. The chemical formula for Ambrox is C16 H28 O. Ambrox was therefore born through organic chemistry in the 1950s at the laboratories of aroma-chemical producing firm Firmenich SA as a substitute for ambergris (grey amber) which was very expensive for wide use in fragrances and exceedingly scarce. (You can read more on ambergris and its commonalities/differences with the term amber on this article). Although used interchangeably with Ambroxan as they share almost identical odour profile, they are not one and the same. The construction of Ambrox follows the route of sclareol, a product of the process of clary sage, a natural essence known to aromatherapists for many years [source]. Nevertheless another path exists for Ambrox synthesis, this time from labdanoic acid, since 2002. The main diterpenoid of the acid fraction of non-polar extracts of Cistus ladaniferus L.) converts using an organoselenium reagent, is then oxidatively degradated in its side chain, and finally cyclization of the resulting tetranorlabdan-8α,12-diol happens. Thus, Ambrox is obtained by a six-step procedure in 33% overall yield from methyl labdanolate. [source] Other paths include synthesis from (E) Nerolidol and β-ionone, as well as through (+) -carvone and thujone. [source]
As is usual on Perfume Shrine when dissecting perfumery materials (this is the list with the posts on them) we revert to a little Chemistry 101 to explain Ambrox and its smell in detail. The chemical formula for Ambrox is C16 H28 O. Ambrox was therefore born through organic chemistry in the 1950s at the laboratories of aroma-chemical producing firm Firmenich SA as a substitute for ambergris (grey amber) which was very expensive for wide use in fragrances and exceedingly scarce. (You can read more on ambergris and its commonalities/differences with the term amber on this article). Although used interchangeably with Ambroxan as they share almost identical odour profile, they are not one and the same. The construction of Ambrox follows the route of sclareol, a product of the process of clary sage, a natural essence known to aromatherapists for many years [source]. Nevertheless another path exists for Ambrox synthesis, this time from labdanoic acid, since 2002. The main diterpenoid of the acid fraction of non-polar extracts of Cistus ladaniferus L.) converts using an organoselenium reagent, is then oxidatively degradated in its side chain, and finally cyclization of the resulting tetranorlabdan-8α,12-diol happens. Thus, Ambrox is obtained by a six-step procedure in 33% overall yield from methyl labdanolate. [source] Other paths include synthesis from (E) Nerolidol and β-ionone, as well as through (+) -carvone and thujone. [source]
Ambrox is typically used as one of the base notes of perfume compositions, due to its extremely lasting velvety effect which oscillates between an impression of ambergris (salty, smooth, skin-like), creamy musky & labdanum-like (read on labdanum on this link) and with "clean"/blond woody facets in the mix too. In short, a fascinating molecule that presents itself as a prism through which different facets can shine. Its reception is undoubtedly one of positive response: You're hit with something that smells warm, oddly mineral and sweetly inviting, yet it doesn't exactly smell like a perfumery or even culinary material. It's perfectly abstract, approximating a person's aura rather than a specific component, much like some of the more sophisticated musk components do. Fittingly, Ambrox solves some of the shortcomings of the latest IFRA restrictions on several musks and animal-like base notes. No wonder it's been used so much in perfumes in the last couple of decades! Although one might argue that synthetics replicate naturals due to increasing constrictions on formula costs on the part of perfume companies, the truth is Ambrox is relatively costly in the mostly inexpensive world of synthetics. However until recently companies were reticent into mentioning its inclusion in a perfume formula. It took the pioneering guts of Geza Schoen and his niche brand Escentric Molecules to elevate chemistry into the realm of bottling single molecules in bottles to be put on one's vanity or bathroom shelf: Molecule 02, solely an Ambroxan dilution was coupled with Molecule 01 fed on only Iso-E Super (details on that material on this link).
Recently companies however took on a different path, actually boasting on their flamboyant, mono-chromatic use of this popular material, thus making ultra-hip Parisian concept-store Colette’s newsletter talk about "fragrances fed on Ambrox"! 2010 might well be the year of Ambrox as apart from Juliette has a Gun who boasts on their sole use of Ambrox diluted in ethanol for their Not a Perfume, other companies bravely declare the emphasis on this synthetic: Another 13, from the New York based brand Le Labo and M Mink by Byredo. The latter fragrance uses Ambrox alongside chypry, aromatic and animalic tonalities which are reminiscent of ink.
Whatever you might think of it, we haven't seen the last of Ambrox yet!
List of Perfumes containing perceptible Ambrox/Ambroxan at the base
(Listed in diminishing order of perceptability. NB. The highlighted links lead to reviews/more info):
Not a Perfume by Juliette has a Gun
Molecule 02 by Escentric Molecules
Another 13 by Le Labo
Calamity J by Juliette has a Gun
Mille et Une Roses by Lancôme
Eau de Fleurs de Capucine by Chloé
Light Blue by Dolce & Gabbana
Vetyver by Lanvin
Géranium pour Monsieur by Frédéric Malle
Baie Rose 26 by Le Labo
M Mink by Byredo
White by Lalique
French Lover by Frédéric Malle
Portrait of a Lady by Frédéric Malle
Rumeur by Lanvin
L'Eau d'Issey Goutte sur un Pétale by Issey Miyake
Midnight Poison by Christian Dior
Emporio Armani Diamonds for Men by Armani
Silver Black by Azzaro
1881 Intense pour Homme by Cerruti
Extravagance d'Amarige by Givenchy
Cuir pour homme by Esteban
A Scent Eau de Parfum Florale by Issey Miyake
Please note that another name for Ambroxan is Orcanox, such as mentioned in Etat Libre d'Orange Malaise of the 1970s.
pic via perseus.blog.so-net.ne.jp
.jpg) As is usual on Perfume Shrine when dissecting perfumery materials (this is the list with the posts on them) we revert to a little Chemistry 101 to explain Ambrox and its smell in detail. The chemical formula for Ambrox is C16 H28 O. Ambrox was therefore born through organic chemistry in the 1950s at the laboratories of aroma-chemical producing firm Firmenich SA as a substitute for ambergris (grey amber) which was very expensive for wide use in fragrances and exceedingly scarce. (You can read more on ambergris and its commonalities/differences with the term amber on this article). Although used interchangeably with Ambroxan as they share almost identical odour profile, they are not one and the same. The construction of Ambrox follows the route of sclareol, a product of the process of clary sage, a natural essence known to aromatherapists for many years [source]. Nevertheless another path exists for Ambrox synthesis, this time from labdanoic acid, since 2002. The main diterpenoid of the acid fraction of non-polar extracts of Cistus ladaniferus L.) converts using an organoselenium reagent, is then oxidatively degradated in its side chain, and finally cyclization of the resulting tetranorlabdan-8α,12-diol happens. Thus, Ambrox is obtained by a six-step procedure in 33% overall yield from methyl labdanolate. [source] Other paths include synthesis from (E) Nerolidol and β-ionone, as well as through (+) -carvone and thujone. [source]
As is usual on Perfume Shrine when dissecting perfumery materials (this is the list with the posts on them) we revert to a little Chemistry 101 to explain Ambrox and its smell in detail. The chemical formula for Ambrox is C16 H28 O. Ambrox was therefore born through organic chemistry in the 1950s at the laboratories of aroma-chemical producing firm Firmenich SA as a substitute for ambergris (grey amber) which was very expensive for wide use in fragrances and exceedingly scarce. (You can read more on ambergris and its commonalities/differences with the term amber on this article). Although used interchangeably with Ambroxan as they share almost identical odour profile, they are not one and the same. The construction of Ambrox follows the route of sclareol, a product of the process of clary sage, a natural essence known to aromatherapists for many years [source]. Nevertheless another path exists for Ambrox synthesis, this time from labdanoic acid, since 2002. The main diterpenoid of the acid fraction of non-polar extracts of Cistus ladaniferus L.) converts using an organoselenium reagent, is then oxidatively degradated in its side chain, and finally cyclization of the resulting tetranorlabdan-8α,12-diol happens. Thus, Ambrox is obtained by a six-step procedure in 33% overall yield from methyl labdanolate. [source] Other paths include synthesis from (E) Nerolidol and β-ionone, as well as through (+) -carvone and thujone. [source]Ambrox is typically used as one of the base notes of perfume compositions, due to its extremely lasting velvety effect which oscillates between an impression of ambergris (salty, smooth, skin-like), creamy musky & labdanum-like (read on labdanum on this link) and with "clean"/blond woody facets in the mix too. In short, a fascinating molecule that presents itself as a prism through which different facets can shine. Its reception is undoubtedly one of positive response: You're hit with something that smells warm, oddly mineral and sweetly inviting, yet it doesn't exactly smell like a perfumery or even culinary material. It's perfectly abstract, approximating a person's aura rather than a specific component, much like some of the more sophisticated musk components do. Fittingly, Ambrox solves some of the shortcomings of the latest IFRA restrictions on several musks and animal-like base notes. No wonder it's been used so much in perfumes in the last couple of decades! Although one might argue that synthetics replicate naturals due to increasing constrictions on formula costs on the part of perfume companies, the truth is Ambrox is relatively costly in the mostly inexpensive world of synthetics. However until recently companies were reticent into mentioning its inclusion in a perfume formula. It took the pioneering guts of Geza Schoen and his niche brand Escentric Molecules to elevate chemistry into the realm of bottling single molecules in bottles to be put on one's vanity or bathroom shelf: Molecule 02, solely an Ambroxan dilution was coupled with Molecule 01 fed on only Iso-E Super (details on that material on this link).
Recently companies however took on a different path, actually boasting on their flamboyant, mono-chromatic use of this popular material, thus making ultra-hip Parisian concept-store Colette’s newsletter talk about "fragrances fed on Ambrox"! 2010 might well be the year of Ambrox as apart from Juliette has a Gun who boasts on their sole use of Ambrox diluted in ethanol for their Not a Perfume, other companies bravely declare the emphasis on this synthetic: Another 13, from the New York based brand Le Labo and M Mink by Byredo. The latter fragrance uses Ambrox alongside chypry, aromatic and animalic tonalities which are reminiscent of ink.
Whatever you might think of it, we haven't seen the last of Ambrox yet!
List of Perfumes containing perceptible Ambrox/Ambroxan at the base
(Listed in diminishing order of perceptability. NB. The highlighted links lead to reviews/more info):
Not a Perfume by Juliette has a Gun
Molecule 02 by Escentric Molecules
Another 13 by Le Labo
Calamity J by Juliette has a Gun
Mille et Une Roses by Lancôme
Eau de Fleurs de Capucine by Chloé
Light Blue by Dolce & Gabbana
Vetyver by Lanvin
Géranium pour Monsieur by Frédéric Malle
Baie Rose 26 by Le Labo
M Mink by Byredo
White by Lalique
French Lover by Frédéric Malle
Portrait of a Lady by Frédéric Malle
Rumeur by Lanvin
L'Eau d'Issey Goutte sur un Pétale by Issey Miyake
Midnight Poison by Christian Dior
Emporio Armani Diamonds for Men by Armani
Silver Black by Azzaro
1881 Intense pour Homme by Cerruti
Extravagance d'Amarige by Givenchy
Cuir pour homme by Esteban
A Scent Eau de Parfum Florale by Issey Miyake
Please note that another name for Ambroxan is Orcanox, such as mentioned in Etat Libre d'Orange Malaise of the 1970s.
pic via perseus.blog.so-net.ne.jp
Thursday, October 7, 2010
Mapping Scentscapes: How to Do it
"Perhaps the earliest attempt to make an urban smell map dates back to Paris in the 1790s, when new ideas about both political equality and hygiene combined to send physician Jean-Noël Hallé on a six-mile odor-recording expedition along the banks of the Seine. His map-making technology consisted of nothing more than a notebook and pencil -- and, of course, his nose."
Sissel Tolaas of course doesn't merely rely on antiquated methods. In her quest to olfactorily map urban landscapes (has already mapped Paris, New York City and Mexico City and is currently working on Kansas City). Tolaas however uses Living Flower Technology in situ: Dr. Braja Mookherjee, a scientist at IFF, one of the world's largest fragrance and flavor companies. Mookherjee was obsessed with capturing the exact odor you experience when you put your nose up to, say, a living jasmine flower, rather than relying on an extract, or "absolute," as it's called in the perfumery business. In a paper (pdf) published in 1990 -- the same year IFF trademarked Mookherjee's discovery as "IFF Living Flower Technology" -- Mookherjee described his dissatisfaction with natural oils and extracts"
Writer Nicola Twilley writes in an extensive (and informative) article in the Atlantic: "My scratch-and-sniff maps show how New Yorkers' smell, rather than what. To make them, I extrapolated data from the as-yet-unpublished results of an extensive study that tested the responses of four hundred New Yorkers to sixty-six different smells over a two-year period from March 2005. The experiment was conducted by Andreas Keller and Leslie B. Vosshall at the Laboratory of Neurogenetics and Behavior, The Rockefeller University. "Our main goal was to try to find the difference between different variants in the DNA and different ways that people rank the smells on a seven-point scale from extremely unpleasant to extremely pleasant," Keller said. "We collected our subjects' demographic information just to control for those types of influences."
Nonetheless, that demographic information revealed some fascinating and significant differences in smell perception between men and women, young and old, and different ethnicities. For my map, I chose twelve of Vosshall and Keller's most interesting test smells, from complex natural extracts such as nutmeg and vanilla to single-note synthetic molecules such as octyl acetate, which is the basis for many artificial orange flavors as well as a key ingredient in Chanel No.5."
Sissel Tolaas of course doesn't merely rely on antiquated methods. In her quest to olfactorily map urban landscapes (has already mapped Paris, New York City and Mexico City and is currently working on Kansas City). Tolaas however uses Living Flower Technology in situ: Dr. Braja Mookherjee, a scientist at IFF, one of the world's largest fragrance and flavor companies. Mookherjee was obsessed with capturing the exact odor you experience when you put your nose up to, say, a living jasmine flower, rather than relying on an extract, or "absolute," as it's called in the perfumery business. In a paper (pdf) published in 1990 -- the same year IFF trademarked Mookherjee's discovery as "IFF Living Flower Technology" -- Mookherjee described his dissatisfaction with natural oils and extracts"
Writer Nicola Twilley writes in an extensive (and informative) article in the Atlantic: "My scratch-and-sniff maps show how New Yorkers' smell, rather than what. To make them, I extrapolated data from the as-yet-unpublished results of an extensive study that tested the responses of four hundred New Yorkers to sixty-six different smells over a two-year period from March 2005. The experiment was conducted by Andreas Keller and Leslie B. Vosshall at the Laboratory of Neurogenetics and Behavior, The Rockefeller University. "Our main goal was to try to find the difference between different variants in the DNA and different ways that people rank the smells on a seven-point scale from extremely unpleasant to extremely pleasant," Keller said. "We collected our subjects' demographic information just to control for those types of influences."
Nonetheless, that demographic information revealed some fascinating and significant differences in smell perception between men and women, young and old, and different ethnicities. For my map, I chose twelve of Vosshall and Keller's most interesting test smells, from complex natural extracts such as nutmeg and vanilla to single-note synthetic molecules such as octyl acetate, which is the basis for many artificial orange flavors as well as a key ingredient in Chanel No.5."
Read more on how to map a city scent-wise following the link above.
Wednesday, September 15, 2010
Christophe Laudamiel on How Smell Works
How does our brain pick up scents? Fractally it seems and not even trained noses get everything at once. As acclaimed perfumer Christophe Laudamiel says:
.jpg) "[Smell perception of fragrant molecules] is processed like patches, like facets. And even the best experts can smell only five to eight facets at a given time," explains Laudamiel during his Big Think interview. "For the brain to register a facet, you have to have at least several components for each facet which together are going to give this signature that then you will recognize as coffee. But you won't be able to recognize the different things that you would see in coffee. Some of them, if you take them one-by-one... smell like raw potato, another one is going to smell like smoke, another one like toasted bread, another one like earth, and et cetera." And goes on to add that when creating Aura for the Thierry Mugler coffret he composed for the issue of novel-adapting film "Perfume, Story of a Murderer", asked to recreate the scent of a virgin, "he focused on the scents of milky elements like rice and the soft and velvety scents of water lily and apricot skin. Of course, to add a little human je ne sais quoi to the fragrance, he also added an a few aldehydes, which helped to mimic the notes that our decomposing skin emits regularly".
"[Smell perception of fragrant molecules] is processed like patches, like facets. And even the best experts can smell only five to eight facets at a given time," explains Laudamiel during his Big Think interview. "For the brain to register a facet, you have to have at least several components for each facet which together are going to give this signature that then you will recognize as coffee. But you won't be able to recognize the different things that you would see in coffee. Some of them, if you take them one-by-one... smell like raw potato, another one is going to smell like smoke, another one like toasted bread, another one like earth, and et cetera." And goes on to add that when creating Aura for the Thierry Mugler coffret he composed for the issue of novel-adapting film "Perfume, Story of a Murderer", asked to recreate the scent of a virgin, "he focused on the scents of milky elements like rice and the soft and velvety scents of water lily and apricot skin. Of course, to add a little human je ne sais quoi to the fragrance, he also added an a few aldehydes, which helped to mimic the notes that our decomposing skin emits regularly".
Read the rest of the article on the Big Think link.
Pic from the film "Perfume, Story of a Murderer" via jdnightghobhadi/livjournal
.jpg) "[Smell perception of fragrant molecules] is processed like patches, like facets. And even the best experts can smell only five to eight facets at a given time," explains Laudamiel during his Big Think interview. "For the brain to register a facet, you have to have at least several components for each facet which together are going to give this signature that then you will recognize as coffee. But you won't be able to recognize the different things that you would see in coffee. Some of them, if you take them one-by-one... smell like raw potato, another one is going to smell like smoke, another one like toasted bread, another one like earth, and et cetera." And goes on to add that when creating Aura for the Thierry Mugler coffret he composed for the issue of novel-adapting film "Perfume, Story of a Murderer", asked to recreate the scent of a virgin, "he focused on the scents of milky elements like rice and the soft and velvety scents of water lily and apricot skin. Of course, to add a little human je ne sais quoi to the fragrance, he also added an a few aldehydes, which helped to mimic the notes that our decomposing skin emits regularly".
"[Smell perception of fragrant molecules] is processed like patches, like facets. And even the best experts can smell only five to eight facets at a given time," explains Laudamiel during his Big Think interview. "For the brain to register a facet, you have to have at least several components for each facet which together are going to give this signature that then you will recognize as coffee. But you won't be able to recognize the different things that you would see in coffee. Some of them, if you take them one-by-one... smell like raw potato, another one is going to smell like smoke, another one like toasted bread, another one like earth, and et cetera." And goes on to add that when creating Aura for the Thierry Mugler coffret he composed for the issue of novel-adapting film "Perfume, Story of a Murderer", asked to recreate the scent of a virgin, "he focused on the scents of milky elements like rice and the soft and velvety scents of water lily and apricot skin. Of course, to add a little human je ne sais quoi to the fragrance, he also added an a few aldehydes, which helped to mimic the notes that our decomposing skin emits regularly".Read the rest of the article on the Big Think link.
Pic from the film "Perfume, Story of a Murderer" via jdnightghobhadi/livjournal
Saturday, July 3, 2010
Sony Wafts Welcoming Scents
The electronics giant we all know, Sony, employs a special blend of smelly essences in its stores diffused through scattered electronic devices in order to welcome women and men projecting feelings of ease and quality. The special blend includes essences of vanilla, mandarin, bourbon and other secret ingredients.
 This is what we learn through an article in the ABC News: "Gino Biondi, the chief marketing officer for ScentAir, the company that developed the scent for Sony and makes the diffusers, says the smell of vanilla puts women, typically intimidated by electronics, at ease, while the mandarin denotes class. The bourbon is there for the guys. "It basically enhances the environment for a first great impression," says Biondi, whose company serves everyone from Express clothing to Mandalay Bay Resorts".
This is what we learn through an article in the ABC News: "Gino Biondi, the chief marketing officer for ScentAir, the company that developed the scent for Sony and makes the diffusers, says the smell of vanilla puts women, typically intimidated by electronics, at ease, while the mandarin denotes class. The bourbon is there for the guys. "It basically enhances the environment for a first great impression," says Biondi, whose company serves everyone from Express clothing to Mandalay Bay Resorts".
But scent in general aids consumerism. A study appearing in the Journal of Consumer Research, affirms that scents improve consumers' memory in relation to products, according to scientists at the University of Michigan and Rutgers University. The co-authors Aradhna Krishna, May Lwin and Maureen Morrin claim that scented products perform better in info memory tests vs. non-scented products. "This occurs even though the product scent is not reintroduced at the time of recall, and even when memory is assessed as much as two weeks after product exposure."
Martin Lindstrom, author of "Buyology: The Truth and Lies about How we Buy" gives some examples of how specific odours act subliminally and how they're used: Vanilla is considered comforting due to its evoking breastfeeding milk, therefore "making you feel childish, young, energetic" while wood reflects a back to nature, earthy, solid, classic set of values. On the other hand fruit is summery, thus making people feel "more open-minded, happy and sexual", while lavender affects the heart beat by slowing it down thus making people linger longer in the stores. Cigars and leather are the perfect choice for banks and law firms, apparently, as these odours reflect "conservative values" (supposedly people in power having the money to afford the smell items, I'd presume, so you feel like you're in the hands of authority and successful monetary churning). Several companies from fashion to cars (and even real estate) work with these guidelines in mind and the trend is only going to expand.
Pic via blog.se-nse.net
 This is what we learn through an article in the ABC News: "Gino Biondi, the chief marketing officer for ScentAir, the company that developed the scent for Sony and makes the diffusers, says the smell of vanilla puts women, typically intimidated by electronics, at ease, while the mandarin denotes class. The bourbon is there for the guys. "It basically enhances the environment for a first great impression," says Biondi, whose company serves everyone from Express clothing to Mandalay Bay Resorts".
This is what we learn through an article in the ABC News: "Gino Biondi, the chief marketing officer for ScentAir, the company that developed the scent for Sony and makes the diffusers, says the smell of vanilla puts women, typically intimidated by electronics, at ease, while the mandarin denotes class. The bourbon is there for the guys. "It basically enhances the environment for a first great impression," says Biondi, whose company serves everyone from Express clothing to Mandalay Bay Resorts".But scent in general aids consumerism. A study appearing in the Journal of Consumer Research, affirms that scents improve consumers' memory in relation to products, according to scientists at the University of Michigan and Rutgers University. The co-authors Aradhna Krishna, May Lwin and Maureen Morrin claim that scented products perform better in info memory tests vs. non-scented products. "This occurs even though the product scent is not reintroduced at the time of recall, and even when memory is assessed as much as two weeks after product exposure."
Martin Lindstrom, author of "Buyology: The Truth and Lies about How we Buy" gives some examples of how specific odours act subliminally and how they're used: Vanilla is considered comforting due to its evoking breastfeeding milk, therefore "making you feel childish, young, energetic" while wood reflects a back to nature, earthy, solid, classic set of values. On the other hand fruit is summery, thus making people feel "more open-minded, happy and sexual", while lavender affects the heart beat by slowing it down thus making people linger longer in the stores. Cigars and leather are the perfect choice for banks and law firms, apparently, as these odours reflect "conservative values" (supposedly people in power having the money to afford the smell items, I'd presume, so you feel like you're in the hands of authority and successful monetary churning). Several companies from fashion to cars (and even real estate) work with these guidelines in mind and the trend is only going to expand.
Pic via blog.se-nse.net
Monday, June 21, 2010
Kiehl's Original Musk & Musk 1921 oil: fragrance review
Amidst the plethora of musk fragrances on the market, some stand out as being individual and bearing their own signature. Decades before Serge Lutens came up with the beastly cajole of Muscs Kublaï Khan, the old New York pharmacy of Kiehl's had broken down that bastion with their Musk fragrance. Its voluminous, expanding earthiness will give you a jolt, searching for that hippy relic sitting some pegs below at the cinema or the theatre (or even the amphiteatre, but let's not go there now). The essence of a seriously funky persona which might have been travelling back from an ashram in India or some Goa hot-spot du jour! Alongside personal enlightenment in the 1960s, there came the musks and the patchoulis essences which characterised a whole generation. And it seems Kiehl's was intent on the pulse despite being founded as far back as 1851.
.jpg) The archived date for the introduction of Kiehl's Original Musk is given as 1963. However, the company likes to hint with their Musk 1921 oil in the Essences collection (the "essences" are oils on which are thematically based the Eaux de toilette) that the recipe goes far back, since their pharmacopoia dates to several decades before. But here's the catch: It couldn't have. And the reason is one of science & history coherence. Simply put, the musks contained in the formula did not exist before WWII! Even though naturally derived macrocyclic musks like Muscone and Exaltolide existed before that date, their price was very high (Muscone's still is) and there could never enter the formula of a "drugstore" perfume. Therefore, the now banned nitromusks were the appropriate choice for those purposes. And this gives rise to another point, which explains the prevalence of so many "musk oils" in the market (certainly so in the 1960s and 1970s), especially at the very low end of the deal, such as Bonne Belle Skin Musk and Jovan Musk oil: These musky ingredients were almost insoluble in alcohol, rendering an alcoholic version of a fragrance very difficult. This also answers my own question, in regards to why some musk fragrances circulating today have a moniker of "musk oil" on their brand name, even though they're in alcoholic form, like the wonderfully rich Jean Louis Gady Musk Oil Eau de Toilette or the drugstore cheapie beautie Gosh Musk Oil No.6. The answer is, they are probably referring to a prior oil-based formula and have substituted the -now banned- nitromusks with an alcohol-diffusing musk component or two (after all, the polycyclics Transeolide, Celestolide and Galaxolide are very, very popular in the modern fragrance industry, as attested by our article on the subject linked)
The archived date for the introduction of Kiehl's Original Musk is given as 1963. However, the company likes to hint with their Musk 1921 oil in the Essences collection (the "essences" are oils on which are thematically based the Eaux de toilette) that the recipe goes far back, since their pharmacopoia dates to several decades before. But here's the catch: It couldn't have. And the reason is one of science & history coherence. Simply put, the musks contained in the formula did not exist before WWII! Even though naturally derived macrocyclic musks like Muscone and Exaltolide existed before that date, their price was very high (Muscone's still is) and there could never enter the formula of a "drugstore" perfume. Therefore, the now banned nitromusks were the appropriate choice for those purposes. And this gives rise to another point, which explains the prevalence of so many "musk oils" in the market (certainly so in the 1960s and 1970s), especially at the very low end of the deal, such as Bonne Belle Skin Musk and Jovan Musk oil: These musky ingredients were almost insoluble in alcohol, rendering an alcoholic version of a fragrance very difficult. This also answers my own question, in regards to why some musk fragrances circulating today have a moniker of "musk oil" on their brand name, even though they're in alcoholic form, like the wonderfully rich Jean Louis Gady Musk Oil Eau de Toilette or the drugstore cheapie beautie Gosh Musk Oil No.6. The answer is, they are probably referring to a prior oil-based formula and have substituted the -now banned- nitromusks with an alcohol-diffusing musk component or two (after all, the polycyclics Transeolide, Celestolide and Galaxolide are very, very popular in the modern fragrance industry, as attested by our article on the subject linked)
Smellwise, Kiehl's Musk 1921 (and to a lesser degree the alcoholic Eau de Toilette Original Musk) is indeed close to Muscs Kublaï Khan, albeit a bit rawer and with a muted, hoarse voice instead of the baritone refinement of the Lutens. Compared to another musk fragrance with a certain reputation, Musc Ravageur by Maurice Roucel for F.Malle, it lacks the sweet spice and is a more to the point musk which can be worn by either sex. At the very start, there is also a similarity of Kiehl's Musk with Kouros by Yves Saint Laurent, which fades later on. The beatific darkness is peeking beneath the floral notes and reveals in fine print what the headlines try to conceal: Here is a living, emoting, squirting human being who hasn't really washed well for a while. If you're not absolutely fanatical about sterilisation, you might get the point in the above.
Notes for Kiehl's Original Musk:
Top: Bergamot nectar, orange blossom
Heart: Rose, lily, ylang ylang, neroli
Base: Tonka bean, white patchouli, musk.
Kiehl's now circulates an alcoholic Eau de Toilette Blend No.1 version of their Musk -apart from their famous oil Musk 1921-, which is tamer (probably due to the exclusion of nitromusks), less skanky and somewhat close to White Musk for Men which The Body Shop introduced a couple of seasons ago. It retails for 39$ for 1.7oz. on the site.
The current ownership by L'Oreal probably means that the cosmetic concerns overshadow any potential adherence to old formulae even more pressingly.
Related reading on Perfume Shrine: The Musk Series (ingredients & cultural history), Scented Musketeers: Musk fragrances reviews.
Photo by Robert Mapplethorpe, Thomas Williams 1987 via cegur.com.
.jpg) The archived date for the introduction of Kiehl's Original Musk is given as 1963. However, the company likes to hint with their Musk 1921 oil in the Essences collection (the "essences" are oils on which are thematically based the Eaux de toilette) that the recipe goes far back, since their pharmacopoia dates to several decades before. But here's the catch: It couldn't have. And the reason is one of science & history coherence. Simply put, the musks contained in the formula did not exist before WWII! Even though naturally derived macrocyclic musks like Muscone and Exaltolide existed before that date, their price was very high (Muscone's still is) and there could never enter the formula of a "drugstore" perfume. Therefore, the now banned nitromusks were the appropriate choice for those purposes. And this gives rise to another point, which explains the prevalence of so many "musk oils" in the market (certainly so in the 1960s and 1970s), especially at the very low end of the deal, such as Bonne Belle Skin Musk and Jovan Musk oil: These musky ingredients were almost insoluble in alcohol, rendering an alcoholic version of a fragrance very difficult. This also answers my own question, in regards to why some musk fragrances circulating today have a moniker of "musk oil" on their brand name, even though they're in alcoholic form, like the wonderfully rich Jean Louis Gady Musk Oil Eau de Toilette or the drugstore cheapie beautie Gosh Musk Oil No.6. The answer is, they are probably referring to a prior oil-based formula and have substituted the -now banned- nitromusks with an alcohol-diffusing musk component or two (after all, the polycyclics Transeolide, Celestolide and Galaxolide are very, very popular in the modern fragrance industry, as attested by our article on the subject linked)
The archived date for the introduction of Kiehl's Original Musk is given as 1963. However, the company likes to hint with their Musk 1921 oil in the Essences collection (the "essences" are oils on which are thematically based the Eaux de toilette) that the recipe goes far back, since their pharmacopoia dates to several decades before. But here's the catch: It couldn't have. And the reason is one of science & history coherence. Simply put, the musks contained in the formula did not exist before WWII! Even though naturally derived macrocyclic musks like Muscone and Exaltolide existed before that date, their price was very high (Muscone's still is) and there could never enter the formula of a "drugstore" perfume. Therefore, the now banned nitromusks were the appropriate choice for those purposes. And this gives rise to another point, which explains the prevalence of so many "musk oils" in the market (certainly so in the 1960s and 1970s), especially at the very low end of the deal, such as Bonne Belle Skin Musk and Jovan Musk oil: These musky ingredients were almost insoluble in alcohol, rendering an alcoholic version of a fragrance very difficult. This also answers my own question, in regards to why some musk fragrances circulating today have a moniker of "musk oil" on their brand name, even though they're in alcoholic form, like the wonderfully rich Jean Louis Gady Musk Oil Eau de Toilette or the drugstore cheapie beautie Gosh Musk Oil No.6. The answer is, they are probably referring to a prior oil-based formula and have substituted the -now banned- nitromusks with an alcohol-diffusing musk component or two (after all, the polycyclics Transeolide, Celestolide and Galaxolide are very, very popular in the modern fragrance industry, as attested by our article on the subject linked)Smellwise, Kiehl's Musk 1921 (and to a lesser degree the alcoholic Eau de Toilette Original Musk) is indeed close to Muscs Kublaï Khan, albeit a bit rawer and with a muted, hoarse voice instead of the baritone refinement of the Lutens. Compared to another musk fragrance with a certain reputation, Musc Ravageur by Maurice Roucel for F.Malle, it lacks the sweet spice and is a more to the point musk which can be worn by either sex. At the very start, there is also a similarity of Kiehl's Musk with Kouros by Yves Saint Laurent, which fades later on. The beatific darkness is peeking beneath the floral notes and reveals in fine print what the headlines try to conceal: Here is a living, emoting, squirting human being who hasn't really washed well for a while. If you're not absolutely fanatical about sterilisation, you might get the point in the above.
Notes for Kiehl's Original Musk:
Top: Bergamot nectar, orange blossom
Heart: Rose, lily, ylang ylang, neroli
Base: Tonka bean, white patchouli, musk.
Kiehl's now circulates an alcoholic Eau de Toilette Blend No.1 version of their Musk -apart from their famous oil Musk 1921-, which is tamer (probably due to the exclusion of nitromusks), less skanky and somewhat close to White Musk for Men which The Body Shop introduced a couple of seasons ago. It retails for 39$ for 1.7oz. on the site.
The current ownership by L'Oreal probably means that the cosmetic concerns overshadow any potential adherence to old formulae even more pressingly.
Related reading on Perfume Shrine: The Musk Series (ingredients & cultural history), Scented Musketeers: Musk fragrances reviews.
Photo by Robert Mapplethorpe, Thomas Williams 1987 via cegur.com.
Tuesday, May 18, 2010
Indole & Jasmine: Indolic vs. Non-Indolic or Dirty vs.Clean
Say the word jasmine among perfume circles and expect to see the characterisation of indolic being brandished a lot at no time. Expect to see upturned noses with "indolic" mentioned alongside "fecal" in the same breath. Is jasmine a "dirty" word? Who potty-trained it? These thoughts evolved in our mind as we re-examined the troubled relationship of perfume lovers to the perfumery "king of flowers", especially now that the weather is warm and jasmine vines are flowering like mad; and so decided to take things at the top.
 Indole is often used as the scapegoat for "stinky" smells, but the truth is somewhat different. Originally indole is a portmanteau of the words "indigo" and "oleum", because the chemical substance named indole was first isolated by treatment of the indigo dye with oleum (oil), thus giving rise to indole chemistry.
Indole is often used as the scapegoat for "stinky" smells, but the truth is somewhat different. Originally indole is a portmanteau of the words "indigo" and "oleum", because the chemical substance named indole was first isolated by treatment of the indigo dye with oleum (oil), thus giving rise to indole chemistry.
Indole is an aromatic heterocyclic organic compound which contains a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring (don't worry if you're not great at chemistry, it will all make sense in a second); thus compounds which contain an "indole ring" (sequence of molecules) are accordingly named "indoles".
What does this mean? It smells "weird". But not necessarily of feces or poop, contrary to common knowledge! Organic chemistry on the whole isn't averse to naming names quite literally, especially when it comes to foul-smelling components: Hence we have cadaverine (for cadaver smell), putrescine (for the stench of garbage), skatole (from the Greek σκατό, litterally meaning shit), or butyric acid (the smell of rancid butter from the Greek βούτυρο/butter). No, the nomenclature of organic chemistry is pretty much to the point, which would pose serious doubts as to why leave such an appropriate scent out there with no fitting baptism!
The answer is simple: Pure indole, the one which is indeed present in feces and also in small part present in white flowers (such as jasmine, gardenia, tuberose and orange blossom; but also in honeysuckle and lilac, technically non white) doesn't really smell of poop in isolation. The white crystals of indole (mainly derived from coal tar) contribute to the effect, in tandem with other things (surely both feces and flowers contain myriads of molecules) but not in seperation so much. Isolated indole has a musty, weird moth-ball smell that is a little stale, reminiscent of decay, like something has gone off and you can't really pinpoint what it is. In presence of humidity and musky compounds it can become a little much, reminiscent of the ambience of a...toilet. In a way an "Eros & Thanatos" concept.
It's interesting to note that a common derivative is the amino acid tryptophan, calming neurotransmiter serotonin's percursor and an essential amino acid in the human diet. (It's isolated in caseine which is found in dairy products, but also in chocolate, oats, poultry, pumpking seeds, peanuts, spirulina and several others. Makes seeing food in a whole different way!).
But should the smell direct us into seperating white floral and jasmine fragrances into naturally-derived or not? In short, does an indolic scent indicate we're dealing with a fragrance containing natural jasmine? The answer isn't as easy as all that. It's true that natural jasmine essence, as used in the perfume industry, is dark and narcotic, containing about 2.5% of pure indole. This often gives a "full", lush and intimate ~some say naughty~ effect in the finished compound, making the jasmine "sexier" or "animalic" as described by perfume enthusiasts (the naughty effect is more due to paracresol, reminiscent of horses' smell). Try Serge Lutens' A la Nuit, also his Sarrasins (a different treatment of intimate) or Montale Jasmine Full, and you know what I am talking about. Olene by Diptyque is another one which has a dubious intimate ambience (described by someone as "one bad mama jama of a jasmine"), as does the extrait version of Joy by Jean Patou, sublimated in rosy and musky tones as well, and the heart of Bal a Versailles (flanked by naughty civet). Also try Bruno Acampora Jasmin. However you don't necessarily have to tread on jasmine to get copious amounts of indole either: Try a carnation scent as well: Carthusia Fiori di Capri. It can make walking in the park where dog owners routinely walk their dogs a completely novel experience in perception!
 Nevertheless, the picking up of a poopy smell can't be a compass into actual composition after all: The natural oil requires the processing of tons of flowers raising the cost to 10,000$ per kilo of essence, while it would be perfectly easy to add seperate indole to synthetic substitutes in order to produce a compound that would be closed in value to 10$ per kilo. The difference in pricing is staggering, which explains why natural jasmine oil is today only used in minute amounts in specific extrait de parfum formulae and only there; the rest is mythos and marketing communication of the brands.
Nevertheless, the picking up of a poopy smell can't be a compass into actual composition after all: The natural oil requires the processing of tons of flowers raising the cost to 10,000$ per kilo of essence, while it would be perfectly easy to add seperate indole to synthetic substitutes in order to produce a compound that would be closed in value to 10$ per kilo. The difference in pricing is staggering, which explains why natural jasmine oil is today only used in minute amounts in specific extrait de parfum formulae and only there; the rest is mythos and marketing communication of the brands.
But not all jasmine fragrances need to be indolic either. Try the non-indolic Armani Sensi and Sensi White Notes. Also get a feel for Jasmine White Moss by E.Lauder or Voile de Jasmine by Bulgari. Nothing "dirty" about them whatsoever!
To construct a light, virginal jasmine without indole, the perfumer has several synthetic options (assuming they're not involved in the masochistic effort of taking the natural and getting it fractioned in order to remove the indole). Hedione in copious amounts is the first choice, as it reproduces a greener, dewier version in contrast to the natural jasmine absolute. It's combination (in elevated ratio within the "jasmine base" thus created) with benzyl acetate, benzyl salicylate (for diffusion and tenacity), alpha amyl cinnamaldehyde and linalool produces the desired effect. These jasmine fragrances are easy to wear, lighter in feel, less heady and do not pose the problem of reminding people of impolite (even if necessary) human functions.
The choice between heaven and hell, as they say, is yours!
If you haven't caught on the Perfumery Definitions series till now, please visit:
Related reading on Perfume Shrine: The Jasmine Series, Raw Aroma Materials of Perfumery
photos by Horst P.Horst in collaboration with Dali and Lisa with Harp.
 Indole is often used as the scapegoat for "stinky" smells, but the truth is somewhat different. Originally indole is a portmanteau of the words "indigo" and "oleum", because the chemical substance named indole was first isolated by treatment of the indigo dye with oleum (oil), thus giving rise to indole chemistry.
Indole is often used as the scapegoat for "stinky" smells, but the truth is somewhat different. Originally indole is a portmanteau of the words "indigo" and "oleum", because the chemical substance named indole was first isolated by treatment of the indigo dye with oleum (oil), thus giving rise to indole chemistry.Indole is an aromatic heterocyclic organic compound which contains a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring (don't worry if you're not great at chemistry, it will all make sense in a second); thus compounds which contain an "indole ring" (sequence of molecules) are accordingly named "indoles".
What does this mean? It smells "weird". But not necessarily of feces or poop, contrary to common knowledge! Organic chemistry on the whole isn't averse to naming names quite literally, especially when it comes to foul-smelling components: Hence we have cadaverine (for cadaver smell), putrescine (for the stench of garbage), skatole (from the Greek σκατό, litterally meaning shit), or butyric acid (the smell of rancid butter from the Greek βούτυρο/butter). No, the nomenclature of organic chemistry is pretty much to the point, which would pose serious doubts as to why leave such an appropriate scent out there with no fitting baptism!
The answer is simple: Pure indole, the one which is indeed present in feces and also in small part present in white flowers (such as jasmine, gardenia, tuberose and orange blossom; but also in honeysuckle and lilac, technically non white) doesn't really smell of poop in isolation. The white crystals of indole (mainly derived from coal tar) contribute to the effect, in tandem with other things (surely both feces and flowers contain myriads of molecules) but not in seperation so much. Isolated indole has a musty, weird moth-ball smell that is a little stale, reminiscent of decay, like something has gone off and you can't really pinpoint what it is. In presence of humidity and musky compounds it can become a little much, reminiscent of the ambience of a...toilet. In a way an "Eros & Thanatos" concept.
It's interesting to note that a common derivative is the amino acid tryptophan, calming neurotransmiter serotonin's percursor and an essential amino acid in the human diet. (It's isolated in caseine which is found in dairy products, but also in chocolate, oats, poultry, pumpking seeds, peanuts, spirulina and several others. Makes seeing food in a whole different way!).
But should the smell direct us into seperating white floral and jasmine fragrances into naturally-derived or not? In short, does an indolic scent indicate we're dealing with a fragrance containing natural jasmine? The answer isn't as easy as all that. It's true that natural jasmine essence, as used in the perfume industry, is dark and narcotic, containing about 2.5% of pure indole. This often gives a "full", lush and intimate ~some say naughty~ effect in the finished compound, making the jasmine "sexier" or "animalic" as described by perfume enthusiasts (the naughty effect is more due to paracresol, reminiscent of horses' smell). Try Serge Lutens' A la Nuit, also his Sarrasins (a different treatment of intimate) or Montale Jasmine Full, and you know what I am talking about. Olene by Diptyque is another one which has a dubious intimate ambience (described by someone as "one bad mama jama of a jasmine"), as does the extrait version of Joy by Jean Patou, sublimated in rosy and musky tones as well, and the heart of Bal a Versailles (flanked by naughty civet). Also try Bruno Acampora Jasmin. However you don't necessarily have to tread on jasmine to get copious amounts of indole either: Try a carnation scent as well: Carthusia Fiori di Capri. It can make walking in the park where dog owners routinely walk their dogs a completely novel experience in perception!
 Nevertheless, the picking up of a poopy smell can't be a compass into actual composition after all: The natural oil requires the processing of tons of flowers raising the cost to 10,000$ per kilo of essence, while it would be perfectly easy to add seperate indole to synthetic substitutes in order to produce a compound that would be closed in value to 10$ per kilo. The difference in pricing is staggering, which explains why natural jasmine oil is today only used in minute amounts in specific extrait de parfum formulae and only there; the rest is mythos and marketing communication of the brands.
Nevertheless, the picking up of a poopy smell can't be a compass into actual composition after all: The natural oil requires the processing of tons of flowers raising the cost to 10,000$ per kilo of essence, while it would be perfectly easy to add seperate indole to synthetic substitutes in order to produce a compound that would be closed in value to 10$ per kilo. The difference in pricing is staggering, which explains why natural jasmine oil is today only used in minute amounts in specific extrait de parfum formulae and only there; the rest is mythos and marketing communication of the brands.But not all jasmine fragrances need to be indolic either. Try the non-indolic Armani Sensi and Sensi White Notes. Also get a feel for Jasmine White Moss by E.Lauder or Voile de Jasmine by Bulgari. Nothing "dirty" about them whatsoever!
To construct a light, virginal jasmine without indole, the perfumer has several synthetic options (assuming they're not involved in the masochistic effort of taking the natural and getting it fractioned in order to remove the indole). Hedione in copious amounts is the first choice, as it reproduces a greener, dewier version in contrast to the natural jasmine absolute. It's combination (in elevated ratio within the "jasmine base" thus created) with benzyl acetate, benzyl salicylate (for diffusion and tenacity), alpha amyl cinnamaldehyde and linalool produces the desired effect. These jasmine fragrances are easy to wear, lighter in feel, less heady and do not pose the problem of reminding people of impolite (even if necessary) human functions.
The choice between heaven and hell, as they say, is yours!
If you haven't caught on the Perfumery Definitions series till now, please visit:
- Definition: Lactonic, Creamy, Milky, Butyric
- Definition: Powdery & Dry in Fragrances
- Definition: Resinous & Balsamic
- Definition: Soapy in Fragrances
- Definition: Phenolic, Terpenic, Camphorous
- Definition: Which Material Produces Which Note/Effect?
Related reading on Perfume Shrine: The Jasmine Series, Raw Aroma Materials of Perfumery
photos by Horst P.Horst in collaboration with Dali and Lisa with Harp.
Tuesday, April 27, 2010
Does Lily of the Valley Act as a Sex Attractant?
The first molecule to challenge the notion that women smell more efficiently than men is revealed, having the dubious ~but fascinating too~ privilige of being the one which also activates OR1D2; that's the human olfactory receptor which, besides being found in the human nose, is also expressed in human sperm cells! Yes, sperm cells actually "smell" all right! The molecule is the aldehyde Bourgeonal and it smells of lily of the valley, also known and referenced as muguet.  (Makes you rethink that green Ajax Fête des fleurs you've been using to mop your floor, doesn't it?)
(Makes you rethink that green Ajax Fête des fleurs you've been using to mop your floor, doesn't it?)
So, do the little swimmers go straight for the dip into your lily of the valley lathered and scented, ahem, private parts? (Lily of the valley substitutes have been for long used in soap making). But before you go out of your way to also clear the shelves of your closest perfumery hall off their stock of Diorissimo and Envy, take a moment to think and appreciate the facts:
It's Bourgeonal alone which activates testicular odor receptors [Science, 2003] and not another lily of the valley aromachemical and it was one among 100 ingredients screened for receptor activation abilities. Bourgeonal just happened to be one of them, which doesn't exclude that there may be others, even more capable of the job. German scientist Hanns Hatt is nevertheless so convinced that he wrote a book (in German) touching upon it with the title: "The lily of the valley phenomenon: All about smell and how it affects our lives". (There could be hundreds of applications in either fertility or contraception products in the future, I guess, not to mention a distinct stirring of the market off the pheromones and into muguet territory! Think about that for a moment...).
The beginning of the research on sperm odour receptors predates this: "Dr. Parmentier, working with researchers in Holland and France, accidentally discovered the sperm receptors while looking for genes that help control thyroid hormones. Using a technique called polymerase chain reaction to scare out even shadows of gene activity from tissue samples, they stumbled on a family of receptor genes active in male germ cells, the precursor tissue that matures into sperm". [source]
To revert to bourgeonal, according to the snippet which I found at First Nerve, where the news was posted, "Peter Olsson and Matthias Laska at Linkőping University in Sweden have finally found a molecule that men detect at reliably lower concentrations than women. It’s an aromatic aldehyde called bourgeonal and as we shall see it’s an interesting molecule for other reasons. [...] Bourgeonal is a potent molecular signal that is critical to sperm chemotaxis, In other words, it’s what sperm use to find their way to the egg. So it makes more than a little evolutionary sense that bourgeonal detection is ramped up in men and their gametic representatives". Of course, Dr.Avery Gilbert has the right humorous tone of telling the facts, so go over and take a read.
But where does the attracting ingredient come from, biologically speaking? "Is it the egg itself, or some other part of the female reproductive tract? 'It could be that the egg is releasing an attractant that helps guide the sperm to the egg, but the problem is that we don't yet know whether, in fact, the egg is the source of that attractant,' Donner Babcock says". [source]
Searching around the Internez I found a post from Jenny van Veenen in Perfume Making back from August 2007, in which she tackles exactly bourgeonal for it sperm kinetics capabilities. The VERY interesting fact and with an especially related connection to perfumes is that "the aroma chemical 'Undecanal' (Aldehyde C-11 undecylenic, waxy/fatty/rose/citrus odor) appears to block the effect of Bourgeonal and inhibits the chemo sensory response in sperm cells. " So watch out those aldehydes if you're trying to get pregnant, you might check out the products you use or if you're trying to avoid getting pregnant you might embrace undecanal etc. A brave new world indeed.
More reading: NY Times , Health 24 and 3Sat (in German)
pic from Perfume Making
 (Makes you rethink that green Ajax Fête des fleurs you've been using to mop your floor, doesn't it?)
(Makes you rethink that green Ajax Fête des fleurs you've been using to mop your floor, doesn't it?)So, do the little swimmers go straight for the dip into your lily of the valley lathered and scented, ahem, private parts? (Lily of the valley substitutes have been for long used in soap making). But before you go out of your way to also clear the shelves of your closest perfumery hall off their stock of Diorissimo and Envy, take a moment to think and appreciate the facts:
It's Bourgeonal alone which activates testicular odor receptors [Science, 2003] and not another lily of the valley aromachemical and it was one among 100 ingredients screened for receptor activation abilities. Bourgeonal just happened to be one of them, which doesn't exclude that there may be others, even more capable of the job. German scientist Hanns Hatt is nevertheless so convinced that he wrote a book (in German) touching upon it with the title: "The lily of the valley phenomenon: All about smell and how it affects our lives". (There could be hundreds of applications in either fertility or contraception products in the future, I guess, not to mention a distinct stirring of the market off the pheromones and into muguet territory! Think about that for a moment...).
The beginning of the research on sperm odour receptors predates this: "Dr. Parmentier, working with researchers in Holland and France, accidentally discovered the sperm receptors while looking for genes that help control thyroid hormones. Using a technique called polymerase chain reaction to scare out even shadows of gene activity from tissue samples, they stumbled on a family of receptor genes active in male germ cells, the precursor tissue that matures into sperm". [source]
To revert to bourgeonal, according to the snippet which I found at First Nerve, where the news was posted, "Peter Olsson and Matthias Laska at Linkőping University in Sweden have finally found a molecule that men detect at reliably lower concentrations than women. It’s an aromatic aldehyde called bourgeonal and as we shall see it’s an interesting molecule for other reasons. [...] Bourgeonal is a potent molecular signal that is critical to sperm chemotaxis, In other words, it’s what sperm use to find their way to the egg. So it makes more than a little evolutionary sense that bourgeonal detection is ramped up in men and their gametic representatives". Of course, Dr.Avery Gilbert has the right humorous tone of telling the facts, so go over and take a read.
But where does the attracting ingredient come from, biologically speaking? "Is it the egg itself, or some other part of the female reproductive tract? 'It could be that the egg is releasing an attractant that helps guide the sperm to the egg, but the problem is that we don't yet know whether, in fact, the egg is the source of that attractant,' Donner Babcock says". [source]
Searching around the Internez I found a post from Jenny van Veenen in Perfume Making back from August 2007, in which she tackles exactly bourgeonal for it sperm kinetics capabilities. The VERY interesting fact and with an especially related connection to perfumes is that "the aroma chemical 'Undecanal' (Aldehyde C-11 undecylenic, waxy/fatty/rose/citrus odor) appears to block the effect of Bourgeonal and inhibits the chemo sensory response in sperm cells. " So watch out those aldehydes if you're trying to get pregnant, you might check out the products you use or if you're trying to avoid getting pregnant you might embrace undecanal etc. A brave new world indeed.
More reading: NY Times , Health 24 and 3Sat (in German)
pic from Perfume Making
Tuesday, December 22, 2009
Musk Series 2: The Natural and Everything about Synthetic Musks
The beauty of musk's scent is that when you smell it on a person's skin, it's hard to tell where one aroma ends and the other begins. "I can feel you on my skin", says one lover to another...The fascinating subject of musk, its origin, its synthetic replication and the multiple variations, surfaces from time to time when we wonder what is hidden in our favourite fragrances ~and not only...
 "Not limited to strong perfumes of questionable taste, musk is in fact the basic ingredient of practically all perfumes, from the most expensive and refined French florals to the sleaziest reek of high school hoochies. Everything in your medicine cabinet contains musk: soaps, shampoos, powders, cosmetics, bath oils, even your toothpaste. It is an ingredient in household cleansers, laundry detergents, insect repellents, and almost every other commercial product that requires fragrance - including food. Does the label say "artificially flavored?" Musk is added to fruit flavors, vanilla, chocolates, licorice, hard candy, chewing gum..."[1]
"Not limited to strong perfumes of questionable taste, musk is in fact the basic ingredient of practically all perfumes, from the most expensive and refined French florals to the sleaziest reek of high school hoochies. Everything in your medicine cabinet contains musk: soaps, shampoos, powders, cosmetics, bath oils, even your toothpaste. It is an ingredient in household cleansers, laundry detergents, insect repellents, and almost every other commercial product that requires fragrance - including food. Does the label say "artificially flavored?" Musk is added to fruit flavors, vanilla, chocolates, licorice, hard candy, chewing gum..."[1]
So where do these musks come from and what are synthetic/white musks?
Short History of Musk Use
Musk is the essence coming from a glandular secretion of the male Tibetan musk deer (Moschus moschiferus L), considered an aphrodisiac (as well as a spiritual fragrance in the Muslim world) in the past. It also fixes and balances a composition , refining it in the most plesasurable and sensuous way and allowing floral and resinous ingredients to flourish in it. Its first historical appearence is in the 5th century BC when it is mentioned in the Talmud (Brachot 43.) as an animal-based fragrance. Despite mentions of the supreme smell of the panther, the creature with the most divine scent imagineable according to the ancient Greeks, no reference per se is made in classical antiquity. In the 6th-century Greek explorer Cosmas Indicopleustes references it as a raw material of Indian origin. It took the Arabs and Byzantine perfume makers for it to rise as an aphrodisiac especiall during the height of the Abbasid Empire. The spice and silk route soon took the aromatic essence aboard and the string of languages that reprised the Sanskrit muṣká (denoting testicle, the source of the musk pod) ~via Middle Persian mušk, Late Greek μόσχος (moschos), Late Latin muscus, Middle French musc and all the way into Middle English muske~ is a journey into its aromatic signposts. In the 1970s musks soared: "In response to the "back-to-nature" ethos of the hippie movement, American perfumers on both coasts sought alternatives to traditional French perfumes. Around 1970, both The Body Shop* in Berkeley, CA, and Kiehl's in New York City introduced single-note "essential oil" fragrances". [2]
*In 1987 The Body Shop was taken over by a huge English firm of the same name, taking over the Musk oil that was synthesized in the 1970s and re-introducing it as White Musk, which is comprised of Galaxolide (7,7%), Tonalide (1,6%) a little Cashmeran (0,1%)for a total of 9.4% of white musks.
Origin and Scent of Natural Deer Musk
The musk deer (moschus moschiferus) is a small, inofensive creature living in Pakistan, India, Tibet, China, Siberia and Mongolia and only the mature male can produce musky odour in rutting season. The best quality musk used to come from Tibet (Tonquin/Tonkin musk) and China, while products from India and Siberia were considered of inferior quality. The practice of extracting the musk pods from the deer however is very difficult, as they're close to the testicles of the animal, and becomes fatally cruel (a kilo of musk necessitated the loss of between 30 to 50 deer), rendering the practice nowadays extinct. (According to Christopher Brosius however , there are currently ways of extracting it without harming the deer being examined ,which could bring back the practice of using real musk). The precious pods (worth twice their weight in gold) were dried in the sun, on hot stones, or by putting them in hot oil. The resulting black granular "musk grain" is used in alcoholic dilution, called tincture.
Natural musk in its raw state is pungent, with a strong pervasive urinous (ammoniac) smell that borders on the somewhat fecal and needs storage and considerable dilution for it to unfold all its potential. Still, The cultural perception of musk varies significantly, often swayed by the smeller's twisted impression of what they are smelling, as discussed in our Musk Series Part 1. Most common descriptors state animalic, earthy and woody notes or baby's skin scent, yet when perfumers talk about musk they refer to muscone, the very core of the musk essence devoid of the other ingredients that are included in natural musk (such as ammonia, cholesterol and animalic compounds with resinous odoriferous characteristics).
.jpg) Other Natural Sources of Musk
Other Natural Sources of Musk
The matter is further complicated by the reference of musk in relation to other animals from which glandular substastances are exracted: Ondatra zibethicus, the muskrat (ondantra zibethicus) a rodent, the Musk Duck (Biziura lobata), the muskox, the musk shrew, the musk beetle (Aromia moschata), African Civet (Civettictis civetta), the musk turtle, the alligator of Central America, and often people refer to the animalics of civet cats (civettictis civetta) or castoreum from beavers as animals' musk, further confusing the terms (those last two animals produce animal substances that have different odour profiles). Even a particular type of alligator emits a musky secretion, but it proved to be non-functional for this type of odour aim as it is mainly rosy.
Some plants also have musky smelling compounds, usually of a lactonic macrocyclic nature, such as Angelica archangelica (containing 12-methyl-13-tridecanolide and Exaltolide) or Abelmoschus moschatus (ambrette seeds) produce musky smelling macrocyclic lactonic compounds which enrich fragrant compositions and are a handy if expensive resource for niche and natural perfumers. The former is amply explored in Angeliques sous la Pluie by Jean Claude Ellena for F.Malle. The latter is highlighted in Chanel Les Exclusifs No.18 and Musc Nomade by Annick Goutal. Other sources include musk flower (Mimulus moschatus), and muskwood (Olearia argophylla), to a much smaller degree. Even galbanum, a bitter green grass not usually associated with milky sweet musky smells, contains musk components (14-pentadecanolide and 15-hexadecanolide).
Interestingly, animal derived musks are all ketones, while plant-derived musks are large-ringed lactones.
The Rise of Synthetic Musks: History and Classification
Musk deers became a protected endangered species by the Convention on the International Trade in Endangered Species of Wild Flora and Fauna (CITES) in 1979, rendering natural musk almost obsolete in perfumery (The erratic quantities used by a handful of perfumers come from either old stock ~musk only gains in complexity with storage and doesn't lose its aroma for centuries~ or illegal poaching). Therefore all musk used in perfumery today is synthetic apart from only a handful exceptions which are usually small artisanal perfumers (who are often not at liberty to be open about it due to ethical concerns from consumers).
Although "white musk" is a prevalent epithet used as a blanket-term, the reality is somewhat more complex. The high cost and scarcity of natural musk had always been a concern for the fragrance industry and it was a happy accidental discovery when in 1888, Baur discovered the nitromusks. He had actually been working on explosives, hence the "nitro" prefix, when he discovered that the molecules actually smelled nice: the warm, sweet ambience recalled the scent of muscone. The first nitromusk was thus baptized Musk Bauer in honour of this occurence and a frantic exploration of these aromatic molecules started with the aim of replicating the elusive smell of natural musk. Follow-up nitro-musks, notably musk ketone and musk xylene, are rich, expansive-smelling and very warm; they can be savoured in vintage extraits and colognes, notably in Chanel No.5 (at a staggering 10% concentration) where they shine with their come-hither whisper. Later developments effectuated different kinds of musks.
Synthetic musks can be therefore divided into three major classes — aromatic nitro musks, polycyclic musk compounds, and macrocyclic musk compounds. The first two groups have broad uses in industry ranging from cosmetics to detergents but their continuous presence in human tissue and environmental samples coupled with carcinogenic properties has created concern resulting in a ban or reduction of their use in most countries. Macrocyclic musk compounds are generally considered safer and have replaced the nitro-musks (abandonded since the early 80s) and slowly phasing out the polycyclics. For those concerned with the dangers, WWF has an interesting article downloadable as PDF on this link. (primarily against musk ketone, musk xylene ~both nitromusks~ and polycyclics)
Synthetic musks are essential in modern perfumery forming the base note of most perfume formulae.

However apart from hydrophibic, polycyclics are also lipophilic, as was first discovered in the early 1990s, building up in the bodies of humans and wildlife over time (Daughton 1999).
Traesolide is another synthetic polycyclic musk used as a fragrance ingredient in a variety of consumer products, including soaps, perfumes, and cosmetics and even though Traseolide is not as commonly used as other polycyclics, such as Galaxolide and Tonalide, it has been detected in breast milk, adipose tissue, and blood in humans (Rimkus 1996; TNO 2004; Duedahl-Olesen 2005). They're being steadily replaced by newer molecules.
Ethylene brassylate (or Musk T) is a brassilic ester with floral woody facets, commonly used in cosmetics, because it acts as an odour neutraliser to the other chemicals used, as well as in fine fragrance. Notably it is featured in Dove's Cleansing Towelettes, as well as Olay, Cover Girl, Max Factor and The Body Shop foundations.
Globalide (Habanolide) is a metallic smelling, fresh radiant musk: Smell it in Emporio Armani White For Her, coupled with Helvetolide (please see below), where it forms the signature of nose Alberto Morillas in 2001, giving rise to the term "white musk" ~as opposed to the balmy darkness of the prior nitromusks. Or try it in the ultra-popular aldehydic musk Glow by Jennifer Lopez, accenting the fresh white floral components of the formula; the cooly herbal-soapy Cologne by Mugler and the baby-soft"clean" of Clair de Musc by Serge Lutens. It also balances the sweeter calorific elements in Hypnotic Poison by Christian Dior.
Ambrettolide is lightly sweetly-musky, uniquely vegetal with bordeline floral tones possessing exceptional diffusion which comes through from the very top notes through the base of the fragrance! Although it naturally occurs in ambrette seeds (prefered by niche brands or natural perfumers), it is usually synthesized in the lab. Other popular macrocyclics are Thibetolide (Exaltolide) ~more detectable by women than by men~ and Velvione, the latter from "velvet" and "ketone", referencing the velvety softness resembling older nitromusks and famously comprising almost the entirety of Helmut Lang's Velvione cologne formula.
One interesting case is IFF's Allyl Amyl Glycolate (iso-amyl oxyacetic acid allylester), one of my less prefered musk variants (Chandler Burr describes it as “a combination of the smell of processed pineapple and the tin of the can it comes in”), a clear liquid that can be used in any blend. It possesses sharp green facets with a top resembling the bitter touch of galbanum and a sweet pineapple fruity note. First discovered in 1936, it lagged unnoticed until it was popularised via Italian detergents in the late 1960s. Its use in Camay soap made it familiar and thus it entered fine perfumery: Trace amounts can be found in Alliage by E.Lauder while higher doses can be found in Drakkar Noir by Guy Laroche (1%)and Cool Water by Davidoff (3% which is very high for a powerful synthetic such as this). Trésor, Eternity, and Boss Elements Aqua also use it for its harmonization with the greener notes (lily of the valley, violet leaf).
.jpg) Newer musks are constantly created, often with imaginative and inspiring names.
Newer musks are constantly created, often with imaginative and inspiring names.
Nirvanolide, a chemical produced by Givaudan has a clean sweetly powdery and slightly animalic odour close to the restricted older Musk Ketone. You can smell it in the perfume Forever Elizabeth created by David Apel where it is used in 6.7% concentration. Another chemical with an odour close to Musk Ketone is Muscenone, possessing a very elegant and diffusive musk odour.
Firmenich offers two musk blends, Auratouch 911382 and Auranone 911383. The base of these products contains a captive* musk with a berry top note that performs like a polycyclic musk. Auratouch 911382 is a strong layered musk base with a substantive drydown and contains only triethyl citrate as a solvent. Meanwhile, the base of Auranone 911383—a strong substantive musk base with a delicate floral and somewhat animalic character—has strong ambrette connotations and contains no solvents, or polycyclic or nitro musks.
Meanwhile, the base of Auranone 911383 is a subtle but substantitve assemblage of some of Firmenich's finest musks with ambrette and animalic facets, blended with soft floral notes, devoid of polycyclic or nitro musks. Created around Romandolide, the captive alicyclic is paired with Habanolide, Exaltolide Total, Muscenone and Helvetolide to produce a hard-core musk with traces of sandalwood, amber, violet and powdery notes.
Givaudan has two new synthetic musks: Cosmone and Serenolide. Cosmone, is a single molecule the first C14-macrocyclic musk commercially available, which has a nitro-musk character of great warmth and diffusion which blends well with all kinds of accords. This biodegradable molecule, in addition to Nirvanolide, enlarges Givaudan’s range of environmentally friendly macrocyclic musks and can be smelled in Pi Neo by Givenchy (2008). Serenolide is an elegant white musk with sweet fruity connotations providing warm and soft velvety notes that blend well with all kinds of trendy fruity accords.
Musk R1 (originally from Quest International) is an example of an oxa-macrolide with sensual, powdery musky character.
The fascinating world of musks is far from over: We will return with classifications, descriptions and reviews of musky fragrances on the market!
*Captives are molecules which are patented by companies for their exclusive use for a number of years.
Related reading on Perfume Shrine: Musk Series 1: a Cultural Perception of Musk, Musk Series 3: The Many Permutations of Musk (musk "types")
Ref:
Rowe, David J. (Ed.); Philip Kraft (2004). "Chapter 7. Aroma Chemicals IV: Musks". Chemistry and Technology of Flavours and Fragrances. Blackwell
Charles (Ed.), Sell; Charles Sell (2005).The Chmistry of Fragrances Chapter 4. Ingredients for the Modern Perfumery Industry". Royal Society of Chemistry Publishing.
Robert R. Calkin and J. Stephen Jellinek, Perfumery Practice & Principles
PChirality & Odour PerceptionJohn C. Leffingwell, Ph.D.
Perfume & Flavorist magazine, Musks in Fragrance Blending
Jenny van Veenen Perfume Making
Aromax blog
[1] [2]Epistola S.Fowler: Musk
Painting Lovers by a Tree Mughal, Muhammad Shah period, about 1725 via lokvani.com. Pics via mikndfully.org, Natural Health Crafters, homotography.blogspot.com
 "Not limited to strong perfumes of questionable taste, musk is in fact the basic ingredient of practically all perfumes, from the most expensive and refined French florals to the sleaziest reek of high school hoochies. Everything in your medicine cabinet contains musk: soaps, shampoos, powders, cosmetics, bath oils, even your toothpaste. It is an ingredient in household cleansers, laundry detergents, insect repellents, and almost every other commercial product that requires fragrance - including food. Does the label say "artificially flavored?" Musk is added to fruit flavors, vanilla, chocolates, licorice, hard candy, chewing gum..."[1]
"Not limited to strong perfumes of questionable taste, musk is in fact the basic ingredient of practically all perfumes, from the most expensive and refined French florals to the sleaziest reek of high school hoochies. Everything in your medicine cabinet contains musk: soaps, shampoos, powders, cosmetics, bath oils, even your toothpaste. It is an ingredient in household cleansers, laundry detergents, insect repellents, and almost every other commercial product that requires fragrance - including food. Does the label say "artificially flavored?" Musk is added to fruit flavors, vanilla, chocolates, licorice, hard candy, chewing gum..."[1]So where do these musks come from and what are synthetic/white musks?
Short History of Musk Use
Musk is the essence coming from a glandular secretion of the male Tibetan musk deer (Moschus moschiferus L), considered an aphrodisiac (as well as a spiritual fragrance in the Muslim world) in the past. It also fixes and balances a composition , refining it in the most plesasurable and sensuous way and allowing floral and resinous ingredients to flourish in it. Its first historical appearence is in the 5th century BC when it is mentioned in the Talmud (Brachot 43.) as an animal-based fragrance. Despite mentions of the supreme smell of the panther, the creature with the most divine scent imagineable according to the ancient Greeks, no reference per se is made in classical antiquity. In the 6th-century Greek explorer Cosmas Indicopleustes references it as a raw material of Indian origin. It took the Arabs and Byzantine perfume makers for it to rise as an aphrodisiac especiall during the height of the Abbasid Empire. The spice and silk route soon took the aromatic essence aboard and the string of languages that reprised the Sanskrit muṣká (denoting testicle, the source of the musk pod) ~via Middle Persian mušk, Late Greek μόσχος (moschos), Late Latin muscus, Middle French musc and all the way into Middle English muske~ is a journey into its aromatic signposts. In the 1970s musks soared: "In response to the "back-to-nature" ethos of the hippie movement, American perfumers on both coasts sought alternatives to traditional French perfumes. Around 1970, both The Body Shop* in Berkeley, CA, and Kiehl's in New York City introduced single-note "essential oil" fragrances". [2]
*In 1987 The Body Shop was taken over by a huge English firm of the same name, taking over the Musk oil that was synthesized in the 1970s and re-introducing it as White Musk, which is comprised of Galaxolide (7,7%), Tonalide (1,6%) a little Cashmeran (0,1%)for a total of 9.4% of white musks.
Origin and Scent of Natural Deer Musk
The musk deer (moschus moschiferus) is a small, inofensive creature living in Pakistan, India, Tibet, China, Siberia and Mongolia and only the mature male can produce musky odour in rutting season. The best quality musk used to come from Tibet (Tonquin/Tonkin musk) and China, while products from India and Siberia were considered of inferior quality. The practice of extracting the musk pods from the deer however is very difficult, as they're close to the testicles of the animal, and becomes fatally cruel (a kilo of musk necessitated the loss of between 30 to 50 deer), rendering the practice nowadays extinct. (According to Christopher Brosius however , there are currently ways of extracting it without harming the deer being examined ,which could bring back the practice of using real musk). The precious pods (worth twice their weight in gold) were dried in the sun, on hot stones, or by putting them in hot oil. The resulting black granular "musk grain" is used in alcoholic dilution, called tincture.
Natural musk in its raw state is pungent, with a strong pervasive urinous (ammoniac) smell that borders on the somewhat fecal and needs storage and considerable dilution for it to unfold all its potential. Still, The cultural perception of musk varies significantly, often swayed by the smeller's twisted impression of what they are smelling, as discussed in our Musk Series Part 1. Most common descriptors state animalic, earthy and woody notes or baby's skin scent, yet when perfumers talk about musk they refer to muscone, the very core of the musk essence devoid of the other ingredients that are included in natural musk (such as ammonia, cholesterol and animalic compounds with resinous odoriferous characteristics).
.jpg) Other Natural Sources of Musk
Other Natural Sources of Musk The matter is further complicated by the reference of musk in relation to other animals from which glandular substastances are exracted: Ondatra zibethicus, the muskrat (ondantra zibethicus) a rodent, the Musk Duck (Biziura lobata), the muskox, the musk shrew, the musk beetle (Aromia moschata), African Civet (Civettictis civetta), the musk turtle, the alligator of Central America, and often people refer to the animalics of civet cats (civettictis civetta) or castoreum from beavers as animals' musk, further confusing the terms (those last two animals produce animal substances that have different odour profiles). Even a particular type of alligator emits a musky secretion, but it proved to be non-functional for this type of odour aim as it is mainly rosy.
Some plants also have musky smelling compounds, usually of a lactonic macrocyclic nature, such as Angelica archangelica (containing 12-methyl-13-tridecanolide and Exaltolide) or Abelmoschus moschatus (ambrette seeds) produce musky smelling macrocyclic lactonic compounds which enrich fragrant compositions and are a handy if expensive resource for niche and natural perfumers. The former is amply explored in Angeliques sous la Pluie by Jean Claude Ellena for F.Malle. The latter is highlighted in Chanel Les Exclusifs No.18 and Musc Nomade by Annick Goutal. Other sources include musk flower (Mimulus moschatus), and muskwood (Olearia argophylla), to a much smaller degree. Even galbanum, a bitter green grass not usually associated with milky sweet musky smells, contains musk components (14-pentadecanolide and 15-hexadecanolide).
Interestingly, animal derived musks are all ketones, while plant-derived musks are large-ringed lactones.
The Rise of Synthetic Musks: History and Classification
Musk deers became a protected endangered species by the Convention on the International Trade in Endangered Species of Wild Flora and Fauna (CITES) in 1979, rendering natural musk almost obsolete in perfumery (The erratic quantities used by a handful of perfumers come from either old stock ~musk only gains in complexity with storage and doesn't lose its aroma for centuries~ or illegal poaching). Therefore all musk used in perfumery today is synthetic apart from only a handful exceptions which are usually small artisanal perfumers (who are often not at liberty to be open about it due to ethical concerns from consumers).
Although "white musk" is a prevalent epithet used as a blanket-term, the reality is somewhat more complex. The high cost and scarcity of natural musk had always been a concern for the fragrance industry and it was a happy accidental discovery when in 1888, Baur discovered the nitromusks. He had actually been working on explosives, hence the "nitro" prefix, when he discovered that the molecules actually smelled nice: the warm, sweet ambience recalled the scent of muscone. The first nitromusk was thus baptized Musk Bauer in honour of this occurence and a frantic exploration of these aromatic molecules started with the aim of replicating the elusive smell of natural musk. Follow-up nitro-musks, notably musk ketone and musk xylene, are rich, expansive-smelling and very warm; they can be savoured in vintage extraits and colognes, notably in Chanel No.5 (at a staggering 10% concentration) where they shine with their come-hither whisper. Later developments effectuated different kinds of musks.
Synthetic musks can be therefore divided into three major classes — aromatic nitro musks, polycyclic musk compounds, and macrocyclic musk compounds. The first two groups have broad uses in industry ranging from cosmetics to detergents but their continuous presence in human tissue and environmental samples coupled with carcinogenic properties has created concern resulting in a ban or reduction of their use in most countries. Macrocyclic musk compounds are generally considered safer and have replaced the nitro-musks (abandonded since the early 80s) and slowly phasing out the polycyclics. For those concerned with the dangers, WWF has an interesting article downloadable as PDF on this link. (primarily against musk ketone, musk xylene ~both nitromusks~ and polycyclics)
Synthetic musks are essential in modern perfumery forming the base note of most perfume formulae.

- Nitro-musks
- Polycyclic musks
However apart from hydrophibic, polycyclics are also lipophilic, as was first discovered in the early 1990s, building up in the bodies of humans and wildlife over time (Daughton 1999).
Traesolide is another synthetic polycyclic musk used as a fragrance ingredient in a variety of consumer products, including soaps, perfumes, and cosmetics and even though Traseolide is not as commonly used as other polycyclics, such as Galaxolide and Tonalide, it has been detected in breast milk, adipose tissue, and blood in humans (Rimkus 1996; TNO 2004; Duedahl-Olesen 2005). They're being steadily replaced by newer molecules.
- Macrocyclic musks
Ethylene brassylate (or Musk T) is a brassilic ester with floral woody facets, commonly used in cosmetics, because it acts as an odour neutraliser to the other chemicals used, as well as in fine fragrance. Notably it is featured in Dove's Cleansing Towelettes, as well as Olay, Cover Girl, Max Factor and The Body Shop foundations.
Globalide (Habanolide) is a metallic smelling, fresh radiant musk: Smell it in Emporio Armani White For Her, coupled with Helvetolide (please see below), where it forms the signature of nose Alberto Morillas in 2001, giving rise to the term "white musk" ~as opposed to the balmy darkness of the prior nitromusks. Or try it in the ultra-popular aldehydic musk Glow by Jennifer Lopez, accenting the fresh white floral components of the formula; the cooly herbal-soapy Cologne by Mugler and the baby-soft"clean" of Clair de Musc by Serge Lutens. It also balances the sweeter calorific elements in Hypnotic Poison by Christian Dior.
Ambrettolide is lightly sweetly-musky, uniquely vegetal with bordeline floral tones possessing exceptional diffusion which comes through from the very top notes through the base of the fragrance! Although it naturally occurs in ambrette seeds (prefered by niche brands or natural perfumers), it is usually synthesized in the lab. Other popular macrocyclics are Thibetolide (Exaltolide) ~more detectable by women than by men~ and Velvione, the latter from "velvet" and "ketone", referencing the velvety softness resembling older nitromusks and famously comprising almost the entirety of Helmut Lang's Velvione cologne formula.
- Alicyclic musks
One interesting case is IFF's Allyl Amyl Glycolate (iso-amyl oxyacetic acid allylester), one of my less prefered musk variants (Chandler Burr describes it as “a combination of the smell of processed pineapple and the tin of the can it comes in”), a clear liquid that can be used in any blend. It possesses sharp green facets with a top resembling the bitter touch of galbanum and a sweet pineapple fruity note. First discovered in 1936, it lagged unnoticed until it was popularised via Italian detergents in the late 1960s. Its use in Camay soap made it familiar and thus it entered fine perfumery: Trace amounts can be found in Alliage by E.Lauder while higher doses can be found in Drakkar Noir by Guy Laroche (1%)and Cool Water by Davidoff (3% which is very high for a powerful synthetic such as this). Trésor, Eternity, and Boss Elements Aqua also use it for its harmonization with the greener notes (lily of the valley, violet leaf).
.jpg) Newer musks are constantly created, often with imaginative and inspiring names.
Newer musks are constantly created, often with imaginative and inspiring names.Nirvanolide, a chemical produced by Givaudan has a clean sweetly powdery and slightly animalic odour close to the restricted older Musk Ketone. You can smell it in the perfume Forever Elizabeth created by David Apel where it is used in 6.7% concentration. Another chemical with an odour close to Musk Ketone is Muscenone, possessing a very elegant and diffusive musk odour.
Firmenich offers two musk blends, Auratouch 911382 and Auranone 911383. The base of these products contains a captive* musk with a berry top note that performs like a polycyclic musk. Auratouch 911382 is a strong layered musk base with a substantive drydown and contains only triethyl citrate as a solvent. Meanwhile, the base of Auranone 911383—a strong substantive musk base with a delicate floral and somewhat animalic character—has strong ambrette connotations and contains no solvents, or polycyclic or nitro musks.
Meanwhile, the base of Auranone 911383 is a subtle but substantitve assemblage of some of Firmenich's finest musks with ambrette and animalic facets, blended with soft floral notes, devoid of polycyclic or nitro musks. Created around Romandolide, the captive alicyclic is paired with Habanolide, Exaltolide Total, Muscenone and Helvetolide to produce a hard-core musk with traces of sandalwood, amber, violet and powdery notes.
Givaudan has two new synthetic musks: Cosmone and Serenolide. Cosmone, is a single molecule the first C14-macrocyclic musk commercially available, which has a nitro-musk character of great warmth and diffusion which blends well with all kinds of accords. This biodegradable molecule, in addition to Nirvanolide, enlarges Givaudan’s range of environmentally friendly macrocyclic musks and can be smelled in Pi Neo by Givenchy (2008). Serenolide is an elegant white musk with sweet fruity connotations providing warm and soft velvety notes that blend well with all kinds of trendy fruity accords.
Musk R1 (originally from Quest International) is an example of an oxa-macrolide with sensual, powdery musky character.
The fascinating world of musks is far from over: We will return with classifications, descriptions and reviews of musky fragrances on the market!
*Captives are molecules which are patented by companies for their exclusive use for a number of years.
Related reading on Perfume Shrine: Musk Series 1: a Cultural Perception of Musk, Musk Series 3: The Many Permutations of Musk (musk "types")
Ref:
Rowe, David J. (Ed.); Philip Kraft (2004). "Chapter 7. Aroma Chemicals IV: Musks". Chemistry and Technology of Flavours and Fragrances. Blackwell
Charles (Ed.), Sell; Charles Sell (2005).The Chmistry of Fragrances Chapter 4. Ingredients for the Modern Perfumery Industry". Royal Society of Chemistry Publishing.
Robert R. Calkin and J. Stephen Jellinek, Perfumery Practice & Principles
PChirality & Odour PerceptionJohn C. Leffingwell, Ph.D.
Perfume & Flavorist magazine, Musks in Fragrance Blending
Jenny van Veenen Perfume Making
Aromax blog
[1] [2]Epistola S.Fowler: Musk
Painting Lovers by a Tree Mughal, Muhammad Shah period, about 1725 via lokvani.com. Pics via mikndfully.org, Natural Health Crafters, homotography.blogspot.com
Labels:
alicyclic musks,
chemistry,
cosmone,
ethylene brassylate,
fragrance science,
galaxolide,
globalide,
macrocyclic musks,
muscenone,
musk deer,
musk series,
nitromusks,
polycyclic musks
Subscribe to:
Comments (Atom)
This Month's Popular Posts on Perfume Shrine
-
Andy Tauer of Tauer Parfums is having his Advent Calendar again this year for the length of December, countring down till Christmas. For the...
-
How many times have you heard that line in one variation or another? Or are you one of the sufferers who feels like you're going to erup...
-
Tauer Perfumes need no introduction: Probably the most successful internet-stemming indie fragrance phenomenon which built sufficient word o...
-
The newest Andy Tauer fragrance, Orange Star , is based on his previous soap-making for Christmas-giving for which he produced Mandarins Amb...
-
Niche perfumer Andy Tauer of Swiss brand Tauer Perfumes has been hosting an Advent Giveaway since December 1st, all the way through December...
-
It was a few days ago that I came forward and announced that Andy Tauer is relaunching one of his less well known perfumes in his line: Eau...
.jpg)





